Method for recycling lithium battery positive electrode material LiCoO2 by ternary eutectic solvent system
A technology of deep eutectic solvent and battery positive electrode, which is applied in the field of battery recycling and deep eutectic solvent, can solve the problems of long leaching time and low efficiency, and achieve the effects of improving leaching efficiency, low recycling cost, and shortening leaching time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
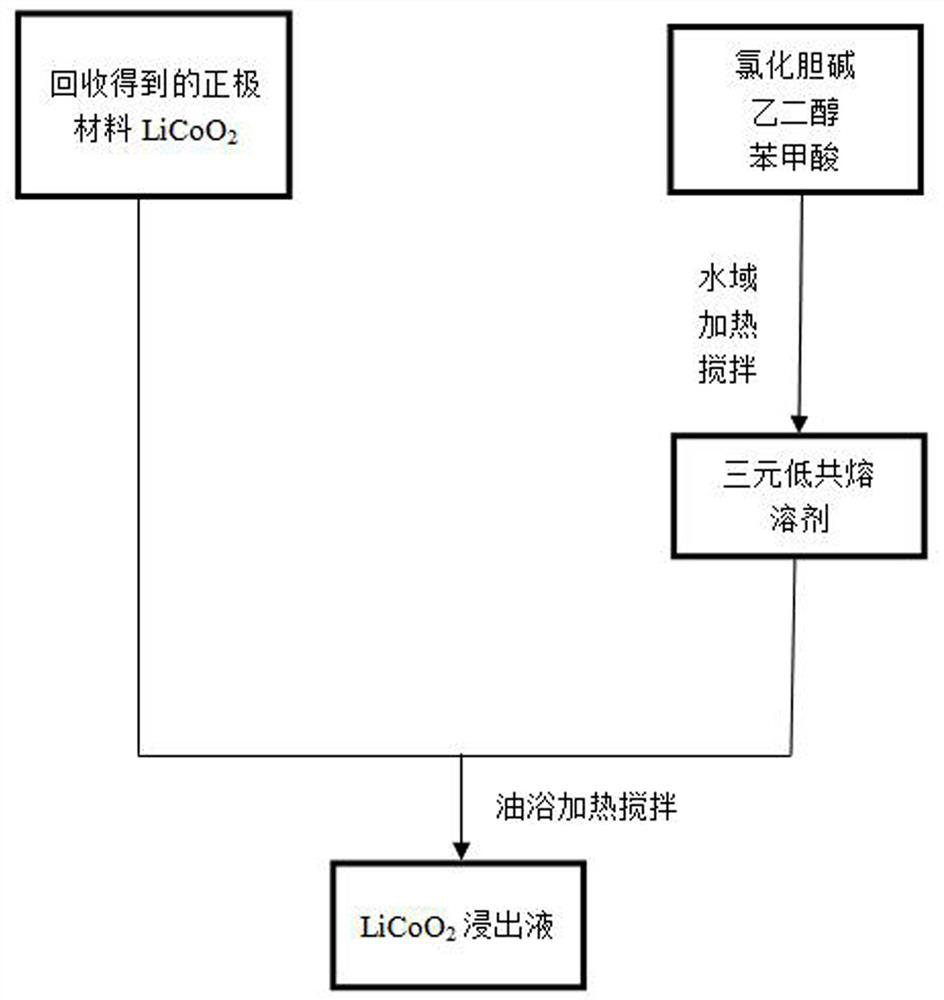
[0037] (1) Preparation of deep eutectic solvent: choline chloride, ethylene glycol and benzoic acid were vacuum-dried at 50°C, then mixed at a molar ratio of 1:1.5:0.5, and mixed at 70°C by heating in a water bath Stir for 2 hours at a stirring rate of 200 rpm / min to obtain a clear and transparent solution.
[0038] (2) Leaching of LiCoO 2 : The recovered LiCoO 2 Added to the prepared DES solution, the added LiCoO 2 The mass ratio to DES is 1:50, and the reaction is carried out in an oil bath at 160°C for 1 hour, and the stirring rate is 400 rpm / min.
[0039] (3) Suction filter the solution obtained in the above steps to obtain a filtrate containing cobalt and lithium ions.
[0040] (4) After testing the concentrations of lithium and cobalt ions in the samples obtained in this example, the leaching rates of lithium and cobalt were calculated to be 93.57% and 98.22%, respectively.
Embodiment 2
[0042] (1) Preparation of deep eutectic solvent: choline chloride, ethylene glycol and benzoic acid were vacuum-dried at 50°C, then mixed at a molar ratio of 1:1.8:0.2, and mixed at 70°C by heating in a water bath Stir for 2 hours at a stirring rate of 200 rpm / min to obtain a clear and transparent solution.
[0043] (2) Leaching of LiCoO 2 : The recovered LiCoO 2Added to the prepared DES, the added LiCoO 2 The mass ratio of DES and DES was 1:5, and the reaction was carried out in an oil bath at 160°C for 2h, and the stirring rate was 400rpm / min.
[0044] (3) Suction filter the solution obtained in the above steps to obtain a filtrate containing cobalt and lithium ions.
[0045] (4) After testing the concentrations of lithium and cobalt ions in the samples obtained in this example, the leaching rates of lithium and cobalt were calculated to be 82.88% and 89.41%, respectively.
Embodiment 3
[0047] (1) Preparation of deep eutectic solvent: choline chloride, ethylene glycol and benzoic acid were vacuum-dried at 50°C, then mixed at a molar ratio of 1:1.5:0.5, and mixed at 70°C by heating in a water bath Stir for 2 hours at a stirring rate of 200 rpm / min to obtain a clear and transparent solution.
[0048] (2) Leaching of LiCoO 2 : The recovered LiCoO 2 Added to the prepared DES, the added LiCoO 2 The mass ratio of DES to DES is 1:50, reacted in an oil bath at 160°C for 2h, and the stirring rate was 400rpm / min.
[0049] (3) Suction filter the solution obtained in the above steps to obtain a filtrate containing cobalt and lithium ions.
[0050] (4) After testing the concentrations of lithium and cobalt ions in the samples obtained in this example, the leaching rates of lithium and cobalt were calculated to be 95.89% and 99.99%, respectively.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com