Preparation method of denitration catalyst
A denitration catalyst and chelating agent technology, applied in chemical instruments and methods, physical/chemical process catalysts, separation methods, etc., can solve problems such as hazards, acid corrosion of catalyst active components, and influence on the adsorption and activation of reactant molecules on the catalyst surface. Achieve the effect of wide catalytic activity temperature window, excellent resistance to alkali metal poisoning, and excellent catalytic activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
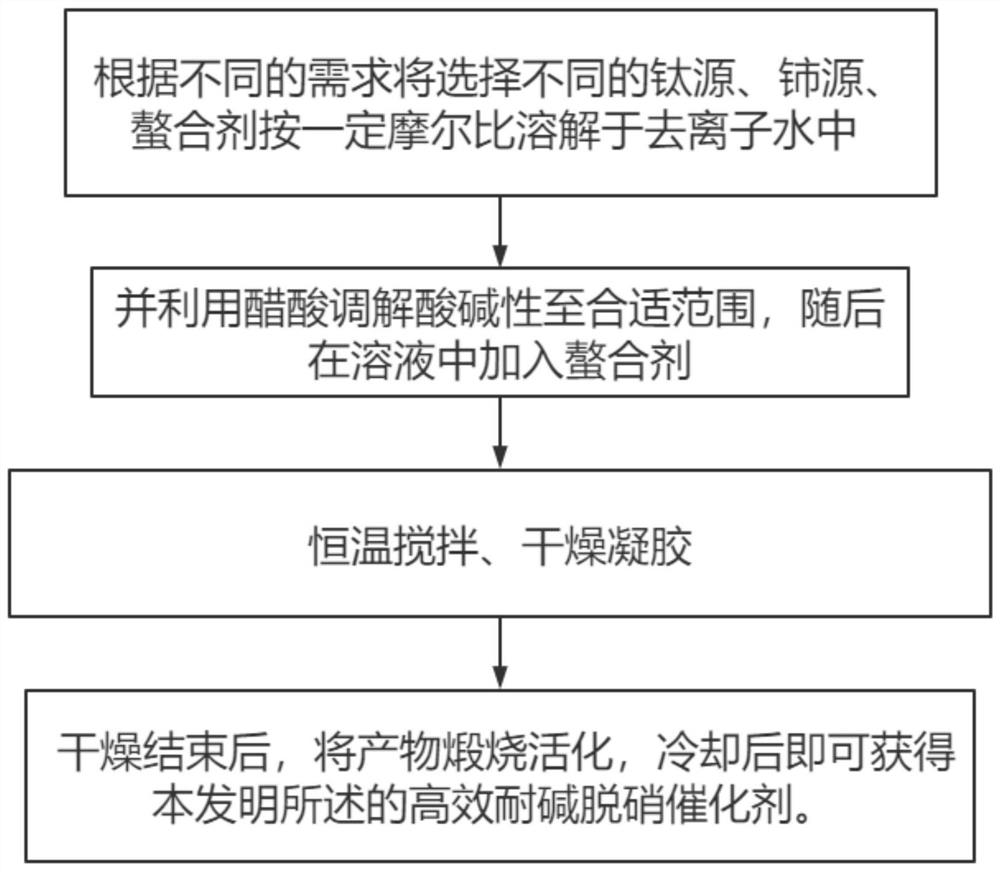
[0022] In view of the problem in the prior art that denitrification catalysts are prone to alkali metal poisoning and deactivation, this application provides a preparation method for denitrification catalysts, which uses a precursor salt containing sulfate groups for the first time to provide sulfate groups in the catalyst. The acidic sites are generated in situ on the surface, and they preferentially combine with the sulfuric acid sites when the alkali metal is poisoned, thereby protecting the original active components and acidic adsorption sites, and avoiding the problem of acid storage, transportation and cleaning in the production process, and solving the problem of Contamination of surface acid residues after catalyst production. Specifically, the embodiment of the present invention discloses a preparation method of a denitration catalyst, comprising the following steps:
[0023] A) dissolving titanium salt and cerium salt in deionized water to obtain a mixed solution; ...
Embodiment 1
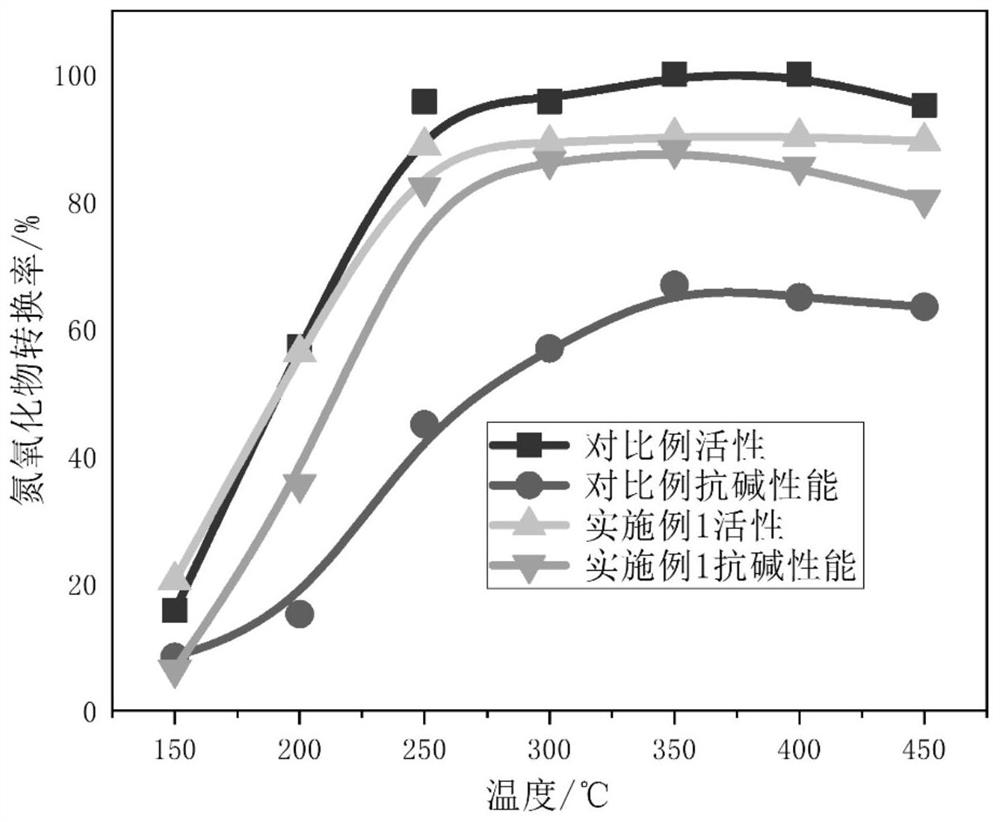
[0032] (1) Catalyst preparation: Weigh titanium sulfate, cerium nitrate and citric acid in a molar ratio of 0.9:0.1:2; first dissolve the cerium nitrate and titanium sulfate in deionized water. After dissolving evenly, add an appropriate amount of acetic acid dropwise to the mixed solution to adjust the pH to 2; then, add citric acid and mix evenly at room temperature; then keep stirring the mixed solution at a constant temperature of 80°C for 8 hours until a sol is formed; The sol was dried at 120°C for 12 hours to obtain a xerogel; after drying, the product was calcined at 450°C for 5 hours, and after cooling, the high-efficiency alkali-resistant denitrification catalyst of the present invention could be obtained.
[0033] (2) Denitrification performance test: Weigh an appropriate amount of catalyst powder prepared above and press it into tablets, sieve it on a 20-mesh to 40-mesh sieve, and put the sieved sample into a catalytic reactor for testing; during the test, 500ppmNO,...
Embodiment 2
[0041] (1) Catalyst preparation: Weigh titanium tetrachloride, cerium sulfate and citric acid in a molar ratio of 0.8:0.2:1.2; first dissolve cerium nitrate and titanium sulfate in deionized water, and after the dissolution is uniform, drop Add an appropriate amount of acetic acid to adjust the pH to 5; then, add citric acid and mix well at room temperature; then keep stirring the mixed solution at a constant temperature of 90°C for 10 hours to form a sol; dry the obtained sol at 100°C for 24 hours to obtain Dry gel; after drying, the product is calcined at 500°C for 2 hours, and after cooling, the high-efficiency alkali-resistant denitrification catalyst of the present invention can be obtained.
[0042] (2) Denitrification performance test: the same as in Example 1.
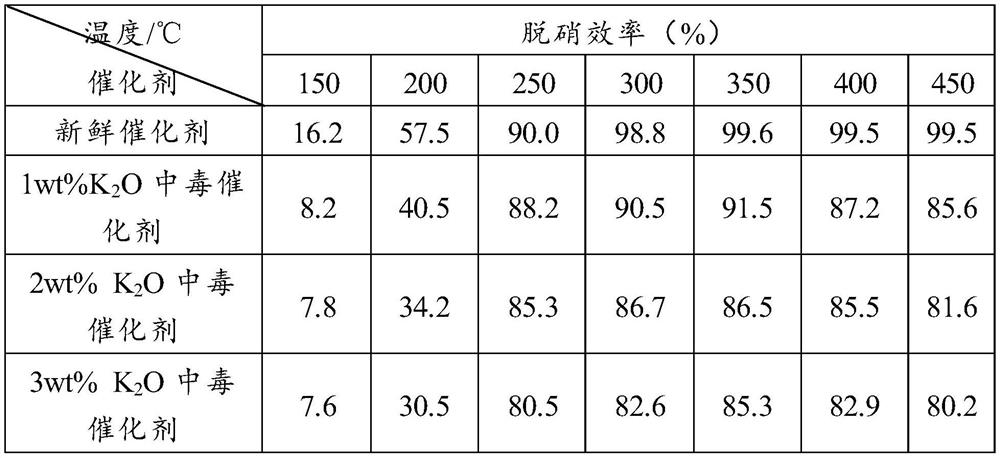
[0043] (3) Preparation of poisoned catalyst: same as in Example 1.
[0044] (4) Alkali resistance test: same as in Example 1.
[0045] Table 2 The denitrification activity of the catalyst prepared in Example ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Bronsted acidity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com