Solid electrolyte and method for producing same
A technology for solid electrolytes and manufacturing methods, applied in solid electrolytes, batteries with solid electrolytes, non-aqueous electrolytes, etc., can solve problems such as the deterioration of sulfide solid electrolytes, and achieve the effects of suppressing the generation of hydrogen sulfide and high ion conductivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
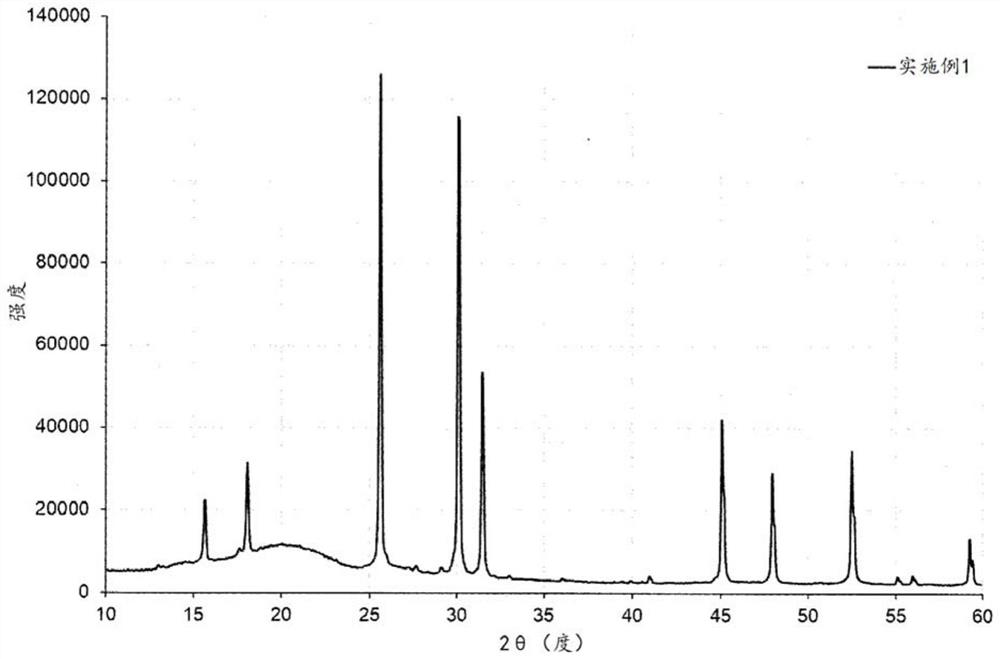
Examples
no. 1 approach
[0036] A method for producing a solid electrolyte according to an embodiment of the present invention includes the following mixing step and heating step, and produces a solid electrolyte having an argentite-type crystal structure.
[0037] Mixing step: a step of mixing raw materials so that lithium (Li), phosphorus (P), sulfur (S), oxygen (O) and halogen (X) satisfy the following formulas (11) to (14).
[0038] 4.8≤Li / P≤5.3...(11)
[0039] 3.8≤S / P≤4.4...(12)
[0040] 0
[0041] 1.0
[0042] (Formula (11) is the molar ratio of Li relative to P, formula (12) is the molar ratio of S relative to P, formula (13) is the molar ratio of O relative to P, formula (14) is the relative molar ratio of halogen (X) to the molar ratio of P.)
[0043] Heating step: a step of heating the mixture obtained in the mixing step.
[0044] In the present embodiment, it is presumed that by adjusting the composition of the starting material to formulas (11) to (14), s...
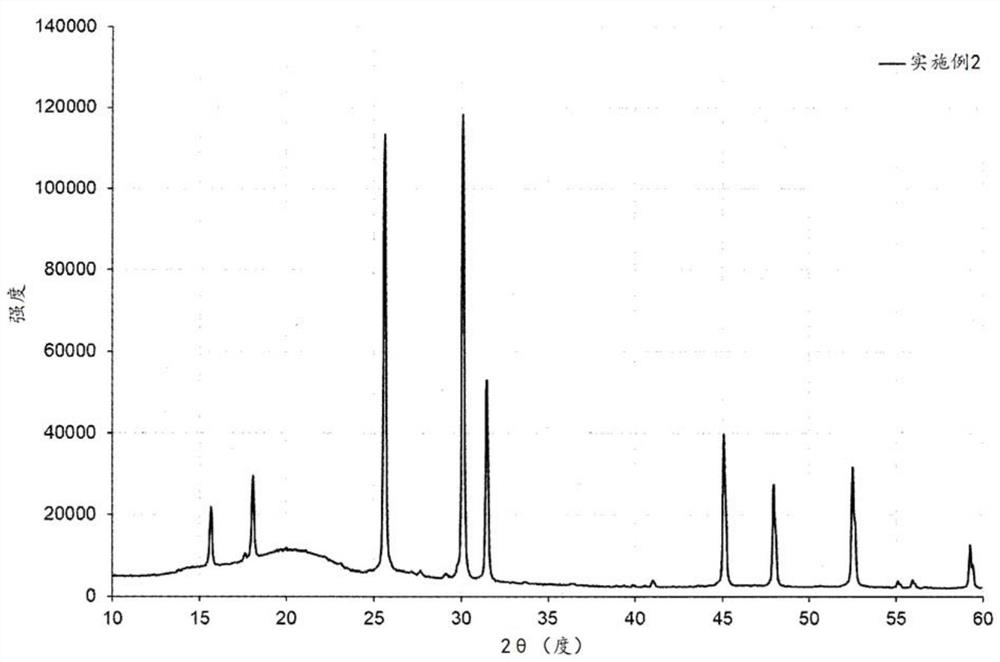
no. 2 approach
[0110] The solid electrolyte of the present embodiment has an argentite-type crystal structure including lithium (Li), phosphorus (P), sulfur (S), oxygen (O) and halogen (X) as constituent elements, and Li in the solid electrolyte 3 PO 4 The ratio of the crystal structure to the total crystals is not less than 0.1% by mass and not more than 3.0% by mass, and the solid electrolyte satisfies the following formulas (21) to (23).
[0111] 4.8≤Li / P≤5.3...(21)
[0112] 3.8≤S / P≤4.4...(22)
[0113] 1.0
[0114] (Formula (21) is the molar ratio of Li relative to P, formula (22) is the molar ratio of S relative to P, and formula (23) is the molar ratio of halogen (X) relative to P.)
[0115] The above formulas (21) to (23) preferably satisfy the following formulas.
[0116] 4.85≤Li / P≤5.25
[0117] 3.9≤S / P≤4.3
[0118] 1.2≤X / P≤1.9
[0119] More preferably, the above formulas (21) to (23) satisfy the following formulas.
[0120] 4.9≤Li / P≤5.2
[0121] 4.0≤S / P≤4.2
...
Embodiment
[0154] Hereinafter, the present invention will be described in more detail based on examples.
[0155] In addition, the evaluation method is as follows.
[0156] (1) Hydrogen sulfide (H 2 S) production volume
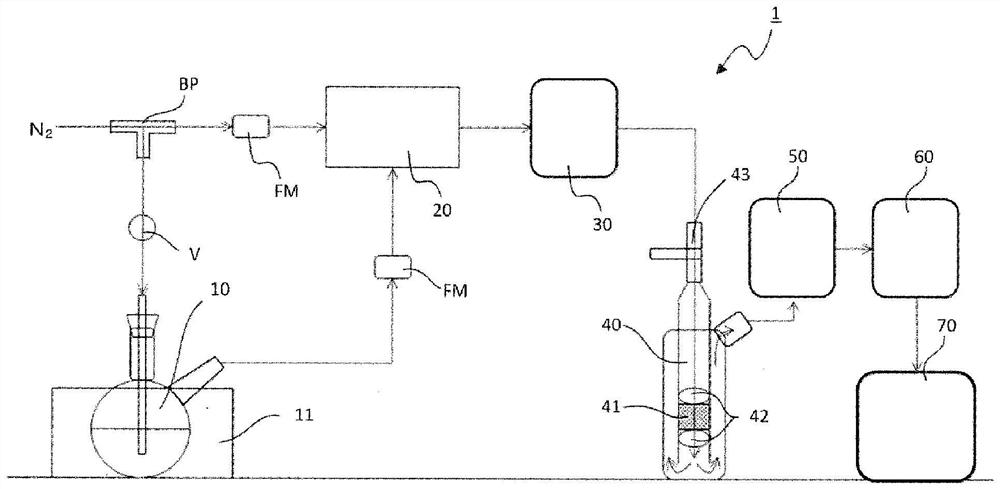
[0157] The schematic diagram of the test device is shown in figure 1 shown.
[0158] The main components of the test device 1 are as follows: a flask 10 for humidifying nitrogen; a static mixer 20 for mixing humidified nitrogen and non-humidified nitrogen; a dew point meter 30 (M170 / DMT152 manufactured by VAISALA Corporation) for measuring the mixed nitrogen double reaction tube 40, set the measurement sample; dew point meter 50, measure the moisture content of the nitrogen gas discharged from the double reaction tube 40; hydrogen sulfide meter 60 (model 3000RS manufactured by AMI Corporation), measure The concentration of hydrogen sulfide is determined, and these constituent elements are connected by pipes (not shown). The temperature of the flask 10 was set to 10...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com