Synthesis method of 2-cyano-4-nitroaniline
A synthetic method and technology of nitroaniline, applied in chemical instruments and methods, preparation of organic compounds, organic chemistry, etc., can solve problems such as not being environmentally friendly, affecting the purity of ammonolysis products, and being difficult to remove
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
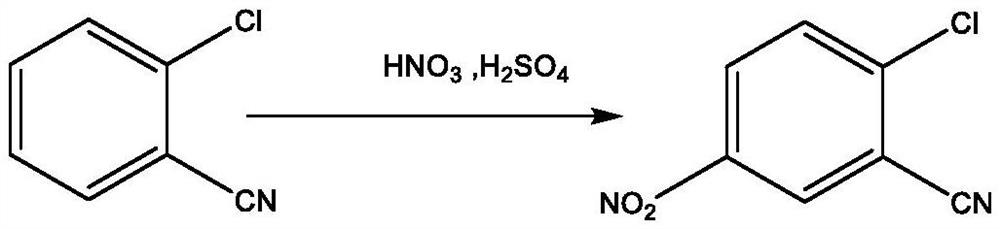
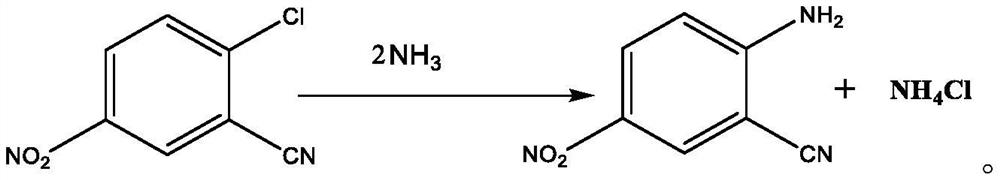
[0045] The invention provides a kind of synthetic method of 2-cyano group-4-nitroaniline, it comprises the following steps:
[0046]Nitration step: dissolving o-chlorobenzonitrile in an organic solvent, and performing a nitration reaction in the presence of a mixed acid to obtain a nitration product, which contains 2-chloro-5-nitrobenzonitrile;
[0047] Mixing step: after mixing the nitrated product with an inorganic solvent, a mixed product is obtained;
[0048] Separation step: statically layering the mixed product to obtain an organic phase;
[0049] Ammonolysis step: passing liquid ammonia and / or ammonia gas into the organic phase to carry out ammonolysis reaction to obtain an ammonolysis product, which contains 2-cyano-4-nitroaniline.
[0050] The method for synthesizing 2-cyano-4-nitroaniline of the present invention saves acid, is environmentally friendly, simplifies operation, and saves costs, while simultaneously ensuring the purity and yield of the product 2-cyano-4...
Embodiment 1
[0079] Nitration reaction: Add 60g of 1,2-dichloroethane into a 250mL four-neck flask, start stirring, slowly drop in 25g of o-chlorobenzonitrile at room temperature, wait until the o-chlorobenzonitrile is completely dissolved, slowly drop in at 5°C Add a mixed acid made of 13g nitric acid and 25g sulfuric acid, drop it for about 1 hour; keep the temperature at 10°C for 2 hours, keep warm, add 90g water slowly, control the acidity value to 22%, then slowly raise the temperature to 45°C, stir 1 hour, and then stood at a temperature of 45°C to separate layers, the lower layer was the 1,2-dichloroethane organic phase, and the upper layer was the inorganic phase. The lower layer was washed with water until neutral (pH value was about 7), the water layer was separated and removed, and transferred to a 500mL autoclave for use.
[0080] Ammonolysis reaction: open the ammonia inlet valve, pour 8g of liquid ammonia into the autoclave, start stirring, raise the temperature to 150°C, con...
Embodiment 2
[0083]Nitration reaction: Add 65g of 1,3-dichloropropane into a 250mL four-necked flask, start stirring, slowly drop in 25g of o-chlorobenzonitrile at room temperature, wait until the o-chlorobenzonitrile is completely dissolved, slowly add Mixed acid made of 13.3g of nitric acid and 20g of sulfuric acid, drop it in about 1 hour; keep the temperature at 10°C for 2 hours, keep warm, add 80g of water slowly, control the acidity value to 20%, slowly raise the temperature to 40°C, stir for 1 hours, and then stood at a temperature of 40°C to separate layers, the lower layer was the 1,3-dichloropropane organic phase, and the upper layer was the inorganic phase. The lower layer was washed with water until neutral (pH value was about 7), the water layer was separated and removed, and transferred to a 500mL autoclave for use.
[0084] Ammonolysis reaction: open the ammonia inlet valve, pour 10g of liquid ammonia into the autoclave, start stirring, raise the temperature to 155°C, contro...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com