Method for synthesizing 1-hydroxyphenothiazine compound
A technology for phenothiazines and compounds, which is applied in the field of synthesizing 1-hydroxyphenothiazine compounds, can solve the problems of high reaction temperature, complicated separation, low yield and the like, and achieves high reaction efficiency, easy availability of synthetic raw materials and wastes. less effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Synthesis of o-benzoquinone:
[0031] Dissolve 1.1g of catechol in 20mL of dichloromethane to form a solution. In a 150mL three-neck flask, put 2.2g of sodium periodate and 50mL of water into it, cool and stir in an ice-water bath to dissolve, quickly add the catechol dichloromethane solution dropwise, continue stirring for 10min, extract 3×15mL of dichloromethane, combine, A solution of o-benzoquinone in dichloromethane was obtained.
[0032] Synthesis of intermediate 2-amino-2',3'-dihydroxydiphenylsulfide:
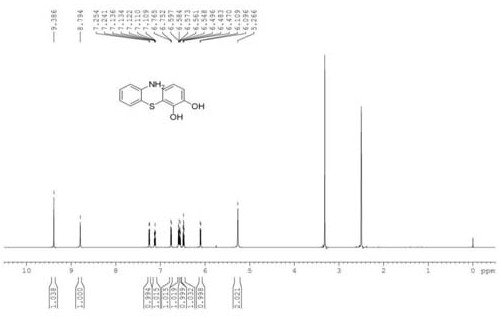
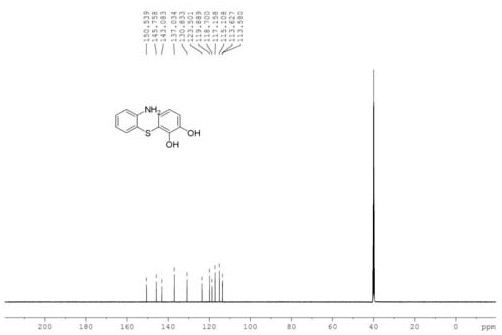
[0033] In a 250 mL three-necked flask, put 1.25 g of o-mercaptoaniline and 50 mL of dichloromethane, stir to dissolve, and add dropwise the o-benzoquinone dichloromethane solution obtained in the previous step. Half of the volume of dichloromethane was removed by rotary evaporation, and an equal volume of petroleum ether was added, and a white solid was precipitated after suction filtration. The solid was recrystallized and dried to obtain 1.67 g of a white soli...
Embodiment 2-11
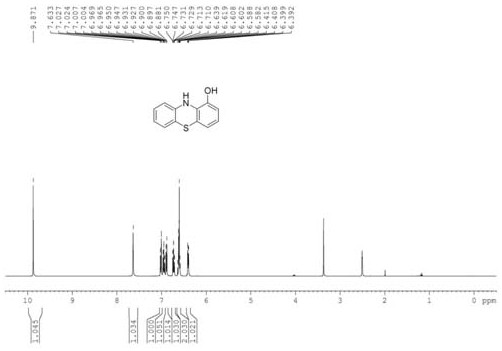
[0039] Synthesize other intermediate diphenyl sulfide compounds by the same method as in Example 1, and the gained intermediate diphenyl sulfide compounds are synthesized by the same method as in Example 1 to other 1-hydroxyphenothiazine compounds, and its various reaction conditions And the reaction results are shown in Table 1.
[0040] Synthesis of various 1-hydroxyphenothiazine compounds under different conditions in table 1
[0041]
[0042]
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com