Citicoline sodium injection and preparation process thereof
A technology of citicoline sodium and injection, which is applied in pharmaceutical formulations, medical preparations with inactive ingredients, and medical preparations containing active ingredients, etc., can solve the problem of low stability of injections and easy deterioration during storage. and other problems, to achieve the effects of excellent stability, reducing storage deterioration and low impurity content
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
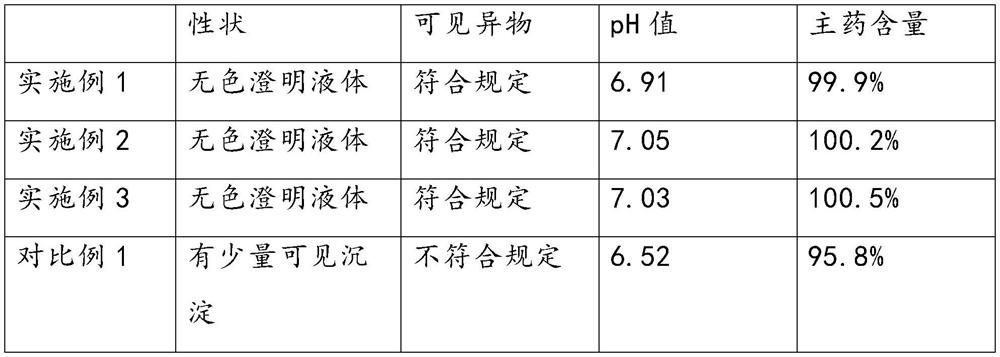
Examples
Embodiment 1
[0023] The present embodiment prepares 980,000 milliliters of citicoline sodium injection, and the specific implementation process is as follows:
[0024] Process S1, ingredients: check that the temperature of the negative pressure weighing room is 18-26°C, the humidity is 45%-65%, the relative pressure difference is ≥-5Pa, the negative pressure weighing hood is opened 20 minutes in advance for self-cleaning, and the medium-effect pressure difference is kept at 50-60Pa, high-efficiency differential pressure maintained at 100-200Pa, then weighed 122.5kg citicoline sodium (in line with QC-STP-160-01 standard), 8.33kg sodium chloride (in line with QC-STP-223-01 standard ) and 98g edetate disodium (conforming to the QC-STP-245-01 standard), measure 980L water for injection (conforming to the QC-STP-215-01 standard), and getting the above raw materials for subsequent use;
[0025] Process S2, concentrated and coarse filtration: check that all equipment containers, various pipes, fi...
Embodiment 2
[0030] The present embodiment prepares a batch of 980,000 milliliters of citicoline sodium injection, and the specific implementation process is as follows:
[0031] Process S1, ingredients: check that the temperature of the negative pressure weighing room is 18-26°C, the humidity is 45%-65%, the relative pressure difference is ≥-5Pa, the negative pressure weighing hood is opened 20 minutes in advance for self-cleaning, and the medium-effect pressure difference is kept at 50-60Pa, high-efficiency differential pressure maintained at 100-200Pa, then weighed 122.5kg citicoline sodium (in line with QC-STP-160-01 standard), 8.33kg sodium chloride (in line with QC-STP-223-01 standard ) and 98g edetate disodium (conforming to the QC-STP-245-01 standard), measure 980L water for injection (conforming to the QC-STP-215-01 standard), and getting the above raw materials for subsequent use;
[0032] Process S2, concentrated and coarse filtration: check that all equipment containers, variou...
Embodiment 3
[0037] This example repeats Example 2 to prepare a batch of 980,000 milliliters of citicoline sodium injection, and the specific formula and process control are exactly the same as those in Example 2.
PUM
| Property | Measurement | Unit |
|---|---|---|
| pore size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com