Use of recombined adenovirus carrying hepatocyte growth factor gene
A technology of hepatocyte growth factor and recombinant adenovirus, which is applied in the field of biomedicine, can solve medical problems, and there are no effective means for the prevention and treatment of fibrous diseases, etc., and achieve a clear effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] Example 1 Construction, amplification and purification of recombinant adenovirus 1. Construction of recombinant adenovirus
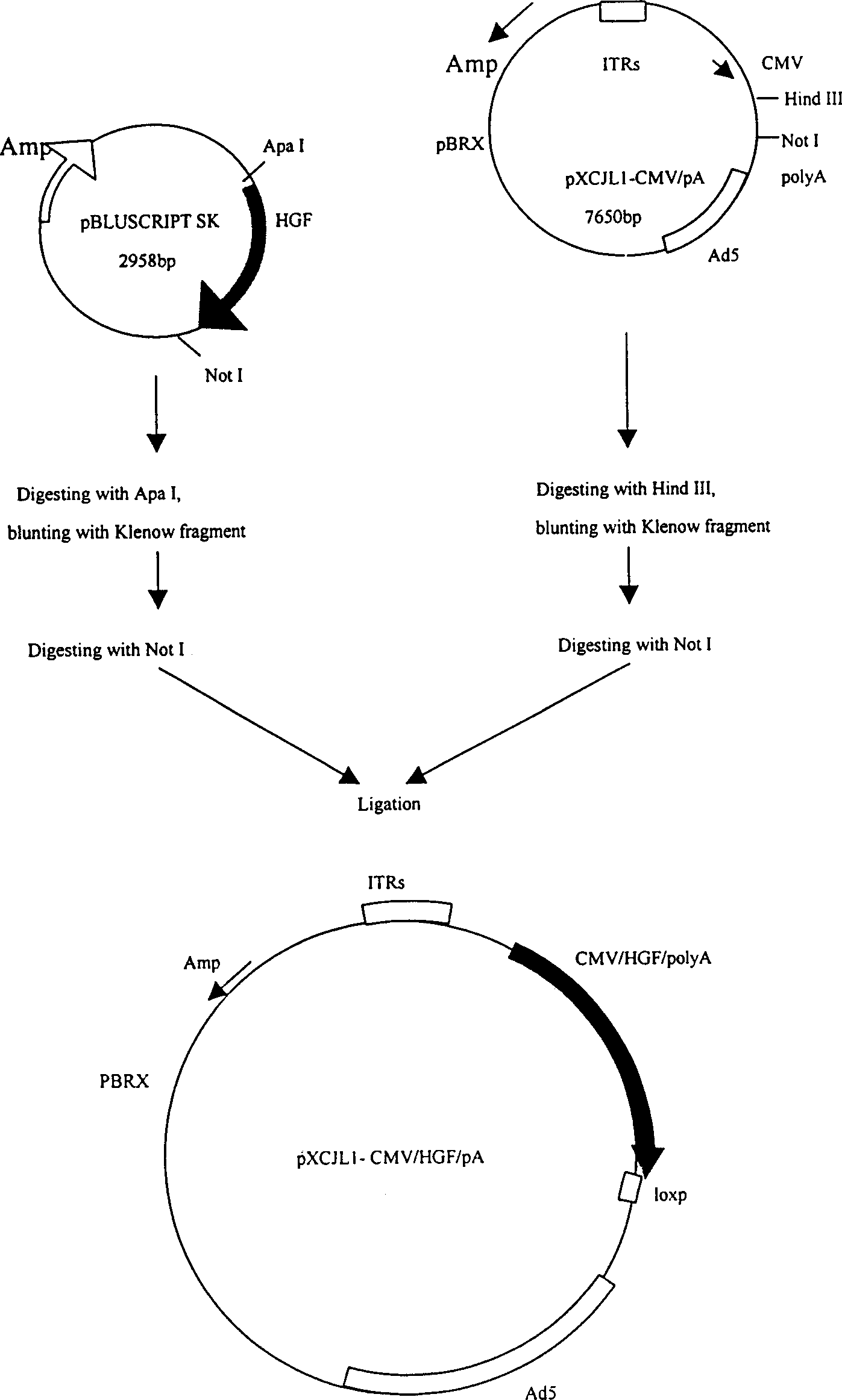
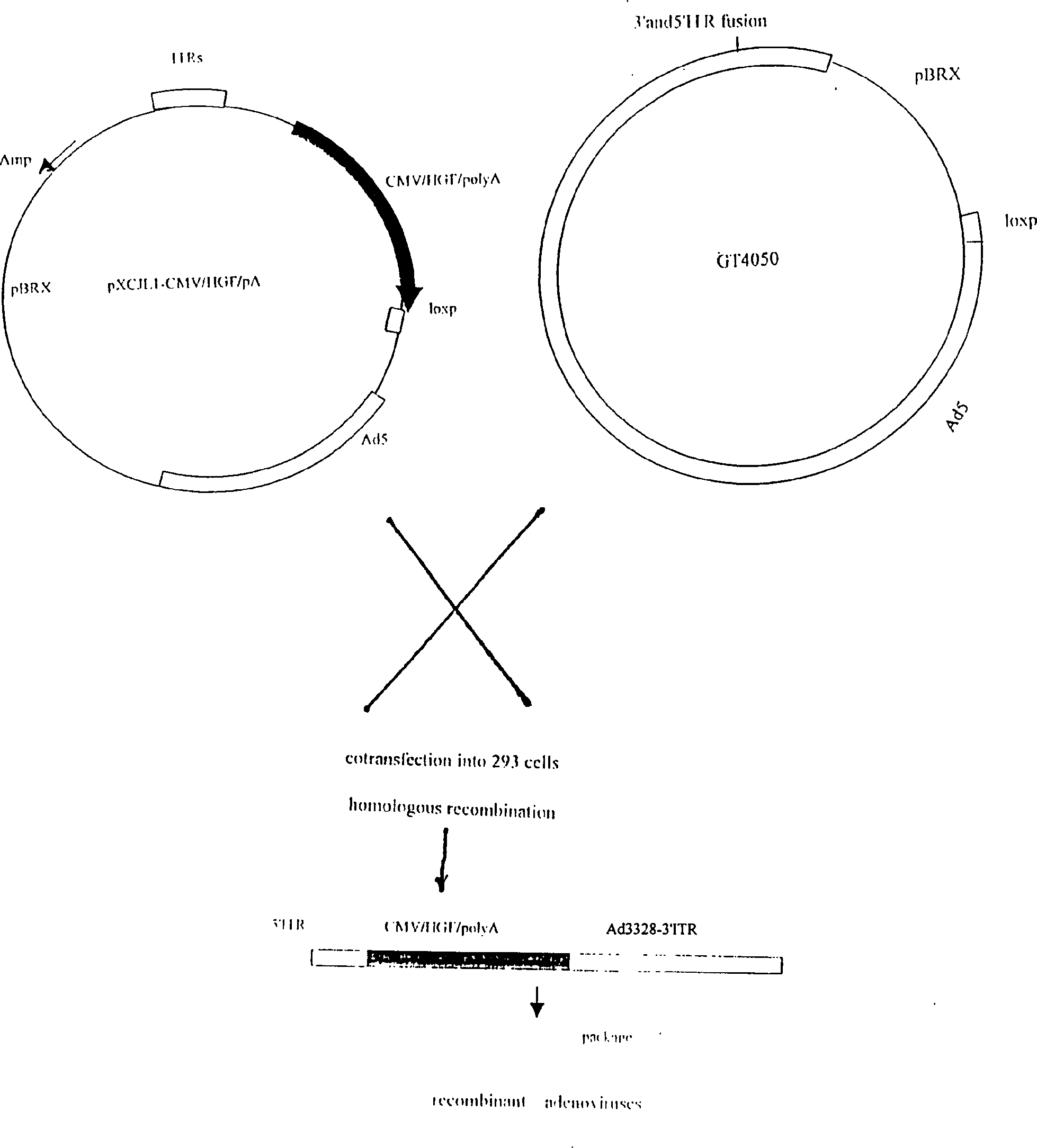
[0033] The construction process of the shuttle plasmid (pXCJL1-CMV / HGF / polyA) carrying the human hepatocyte growth factor gene is as follows: figure 1 shown. 293 cells were co-transfected with pXCJL1-CMV / HGF / polyA and the recombinant plasmid GT4050 carrying the genomic DNA of the adenovirus, and homologous recombination occurred in the cells to form a recombinant adenovirus carrying the human hepatocyte growth factor gene (named as Ad -HGF), the schematic diagram of its structure is shown in figure 2 shown. 2. Preparation and purification of recombinant adenovirus
[0034] 293 cells were inoculated in a 650ml cell culture flask. When the cells grew to 90% confluence, the virus was added in an amount of 10 plaque-forming units (pfu) / cell. After 36-48 hours, the cells showed a complete pathological effect ( CPE), collect the cells and fre...
Embodiment 2
[0035] Example 2 Recombinant adenovirus mediates human hepatocyte growth factor gene
[0036]Evaluation of rat liver fibrosis treatment 1. Preparation of rat liver fibrosis model
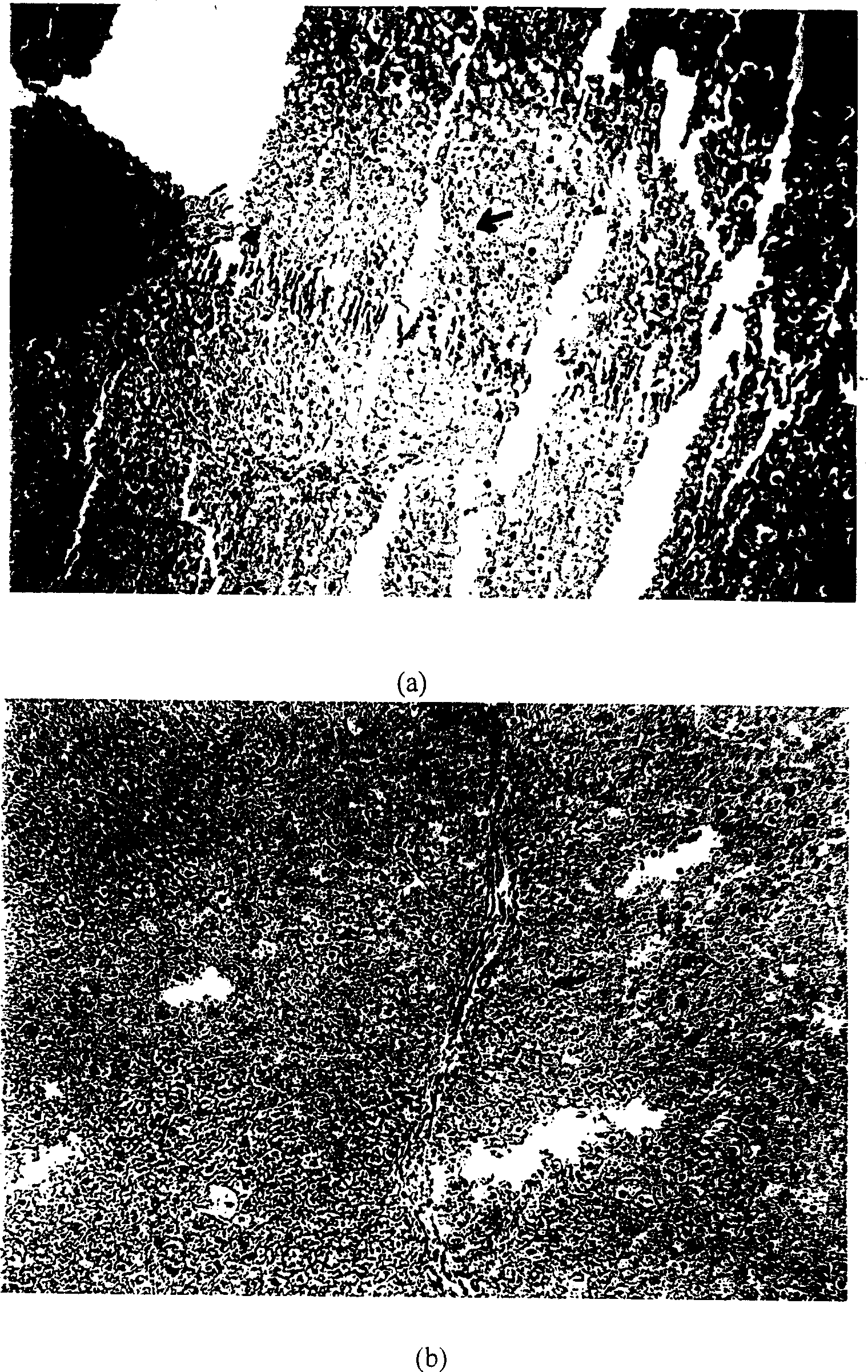
[0037] Thirty Wistar male rats, weighing 200-250 grams. 26 rats were randomly selected to prepare a fibrosis model: 1 g of dinitronitrosamine (DMN) was dissolved in 100 ml of sterile saline to make the final concentration 1%. Rats were administered subcutaneously, 1ml / kg body weight each time, twice a week, for 4 consecutive weeks; the other 4 rats were used as the normal group, and the other conditions were the same as the model group except that no drug was administered. Ten days after the last administration, two rats in each of the model group and the normal group were randomly selected, and the liver tissues were taken for HE section staining to observe the pathological changes in the liver of the rats with liver fibrosis; liver pathological examination: the rats in the model group The...
Embodiment 3
[0039] Example 3 Recombinant adenovirus-mediated human hepatocyte growth factor gene
[0040] Preventive effect on scar formation 1. Scar animal model
[0041] New Zealand female white rabbits, weighing 2.5-3.5kg. After shaving and disinfecting the avascular area on the ventral side of the ear, a trephine was used to microsurgically remove the full-thickness skin until the surface of the cartilage. The diameter of the circular incision was 0.6 cm, and there were 4-6 incisions on each side. 2. Effect evaluation
[0042] Apply the recombinant adenovirus to the wound surface while excising the skin, 8ul each time (8.6×10 7 pfu / μl) (Ad-HGF group), once a day for 3 consecutive days; the wounds of the control group were smeared with the same dose of Ad-GFP. On the 22nd day after the operation, the wound and the adjacent non-wound skin were excised, paraffin sections were made, and HE staining was performed. The results showed that the Ad-HGF application ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com