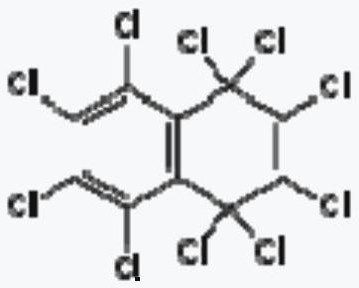
Preparation method of octachloronaphthalene
A technology of chloronaphthalene and naphthol, applied in the field of preparation of octachloronaphthalene, can solve the problems of difficult control of decachloronaphthalene, long reaction time, low yield, etc., and achieves a product content that does not require purification, rapid reaction, and high product content. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] (1) Chlorination stage: add 173g of dichloroethane and 57.67g (0.4mol) of 2-naphthol to the reaction bottle, connect ventilation equipment, stirring device, condenser, thermometer, safety bottle and tail gas absorption device, oil bath After raising the temperature to 85°C, start to ventilate 200g (2.8mol) to react for 8h;
[0029] (2) Hydroxychloride belt stage: Nitrogen gas is passed into the reaction bottle after the completion of chlorine passage to effectively replace residual chlorine gas, then 125g (0.6mol) of phosphorus pentachloride with 1 times the mass of 2-naphthol is added, and the temperature is raised to 90°C , followed by HPLC until the reaction was complete.
[0030] (3) After the reaction was completed, the temperature was lowered to room temperature, and a large amount of solids were precipitated, which were separated by filtration to obtain 148.56 g of the product.
[0031] The yield was 92%, and the HPLC content was 99.1%.
Embodiment 2
[0033] (1) Chlorination stage: add chloroform 865g, 2-naphthol 57.67g (0.4mol) in reaction bottle, connect aeration equipment, stirring device, condenser, thermometer, safety bottle and tail gas absorption device, oil bath is heated up to 70 After ℃, start to ventilate 285g (4mol) to react for 6h;
[0034] (2) Hydroxychloride belt stage: feed nitrogen into the reaction flask after the completion of chlorine passage to effectively replace residual chlorine, then add 164.8g (1.2mol) of phosphorus trichloride with 3 times the mass of 2-naphthol, and heat up to 70 °C, followed by HPLC until the reaction was complete.
[0035] (3) After the reaction was completed, the temperature was lowered to room temperature, and a large amount of solids were precipitated, which were separated by filtration to obtain 145.33 g of the product.
[0036] The yield was 90%, and the HPLC content was 99.2%.
Embodiment 3
[0038] (1) Chlorine passage stage: add tetrachlorethylene 460g, 2-naphthol 57.67g (0.4mol) in reaction bottle, connect ventilation equipment, stirring device, condenser, thermometer, safety bottle and tail gas absorption device, oil bath heats up After reaching 120°C, start to ventilate 227g to react for 4h;
[0039] (2) Hydroxychloride belt stage: Nitrogen gas is passed into the reaction bottle after the completion of chlorine passage to effectively replace the residual chlorine gas, then 92g (0.6mol) of phosphorus oxychloride 1.5 times the amount of 2-naphthol is added, and the temperature is raised to 130°C , followed by HPLC until the reaction was complete.
[0040] (3) After the reaction was completed, the temperature was lowered to room temperature, and a large amount of solids were precipitated, which were separated by filtration to obtain 153.4 g of the product.
[0041] Yield 95%, HPLC content 99%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com