Method for detecting contents of D-ribose and 5-deoxy-D-ribofuranose in capecitabine intermediate
A technology of ribofuranose and capecitabine, applied in the direction of measuring devices, instruments, scientific instruments, etc., to achieve the effects of improving drug safety, good separation effect, and high sensitivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] 1 Instruments and materials
[0030] 1.1 Instrument: triple quadrupole liquid mass spectrometer (Thermo Ultimate 3000-TSQ Quantiva);
[0031] 1.2 Reagents: Acetonitrile is chromatographic grade, and water is ultrapure water.
[0032] 2 Methods and Results
[0033] 2.1 Chromatography and mass spectrometry conditions
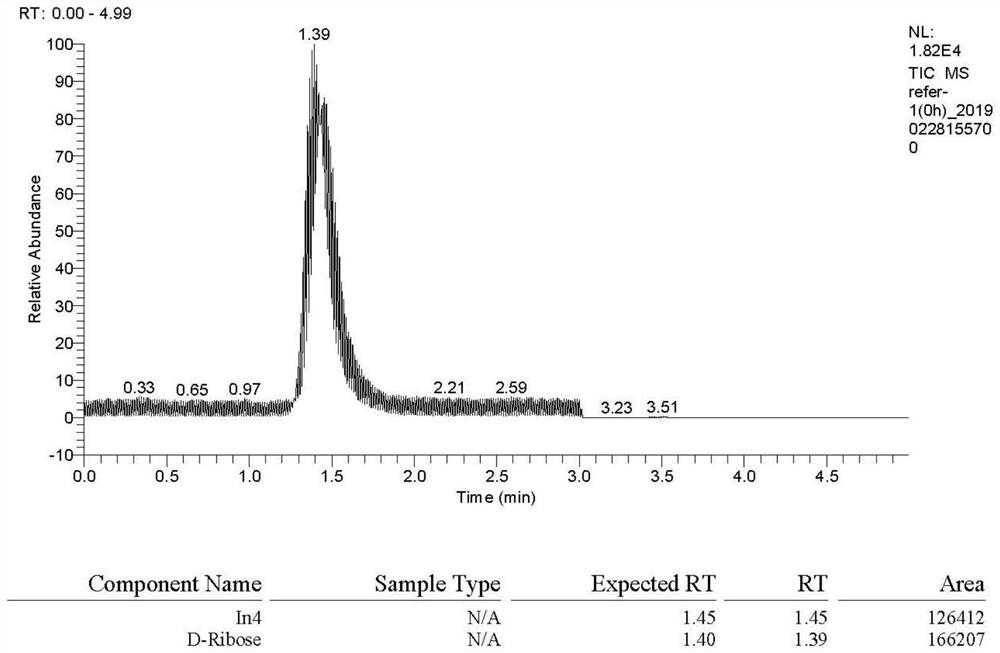
[0034] Chromatographic column: Acclaim RSLC120 C18 (2.0×100mm, 2.2μm), flow rate: 0.2ml / min, column temperature: 20-50℃, injection volume: 2μl, mobile phase: acetonitrile-water (35:65).
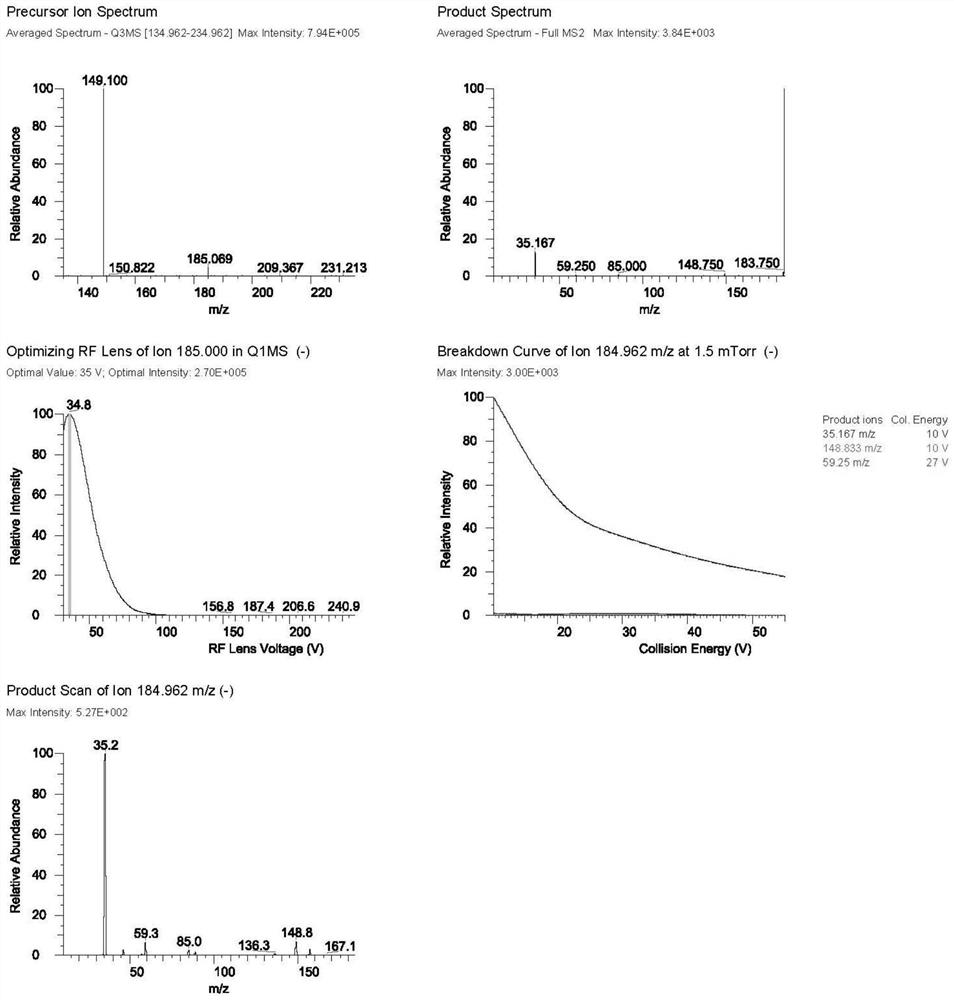
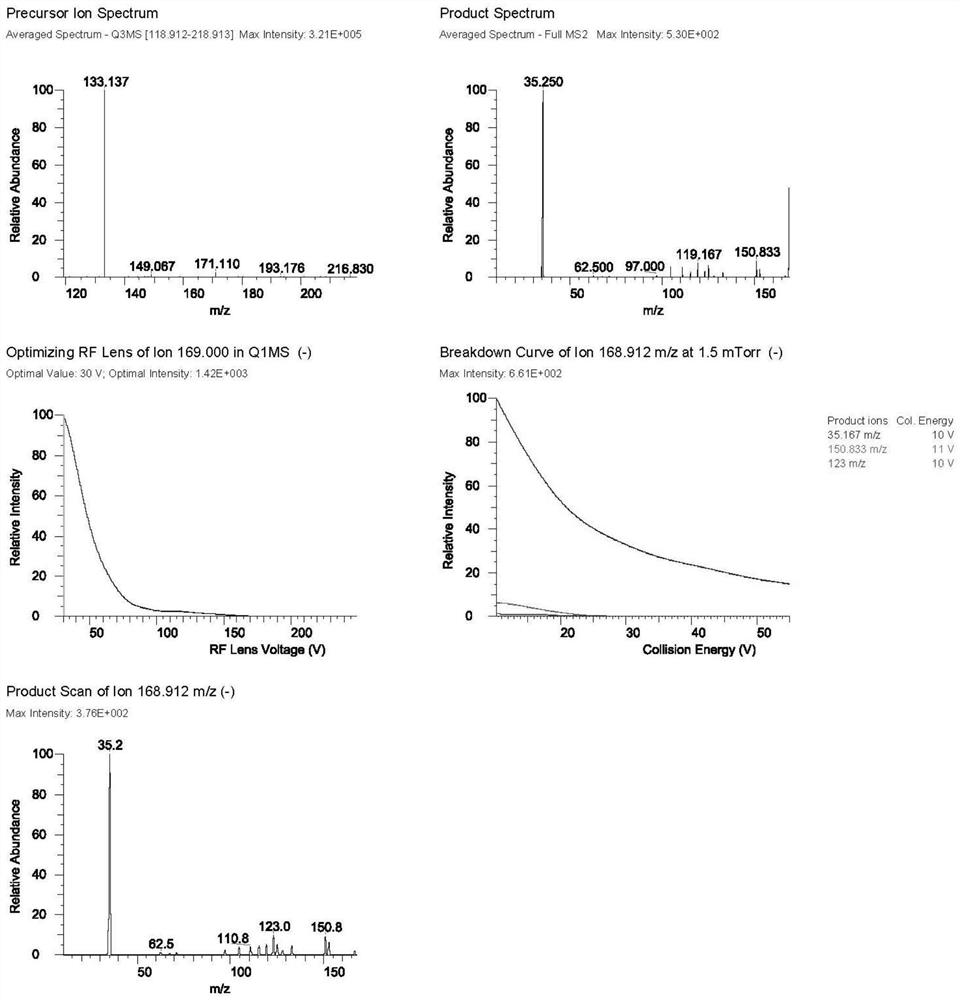
[0035] Mass spectrometry conditions are: ion source: electrospray ionization source (ESI), negative ion mode: selective quantitative (SRM) mode, spray voltage (IS): 2800V; evaporation temperature 200 ° C; sheath gas (Gas1): 35Arb; auxiliary gas (Gas2 ): 7Arb;
[0036] Ion transfer tube temperature (Ion Transfer Tube Temp): 325°C; ion pairs used for quantitative analysis are m / z184.962→m / z35.167 (D-ribose) and m / z168.912→m / z35.250 (5-deoxy-D-ribofuranose), the mass spe...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com