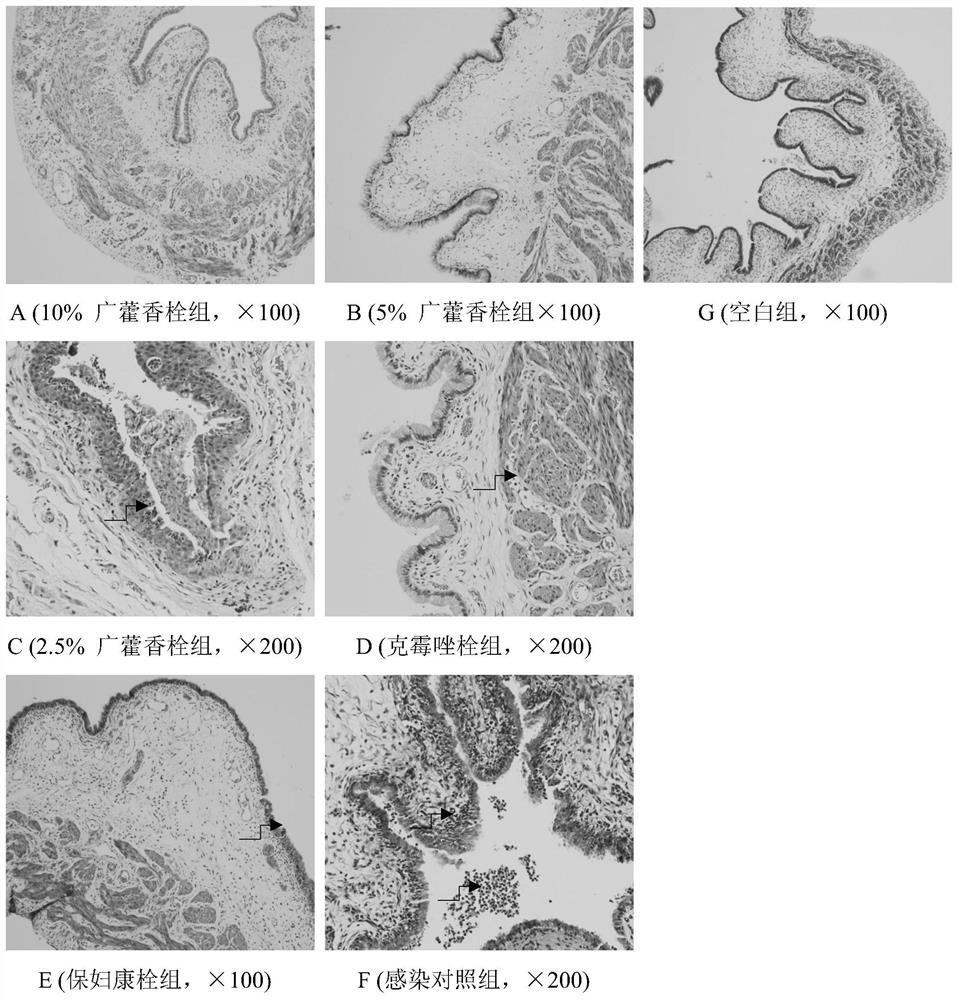
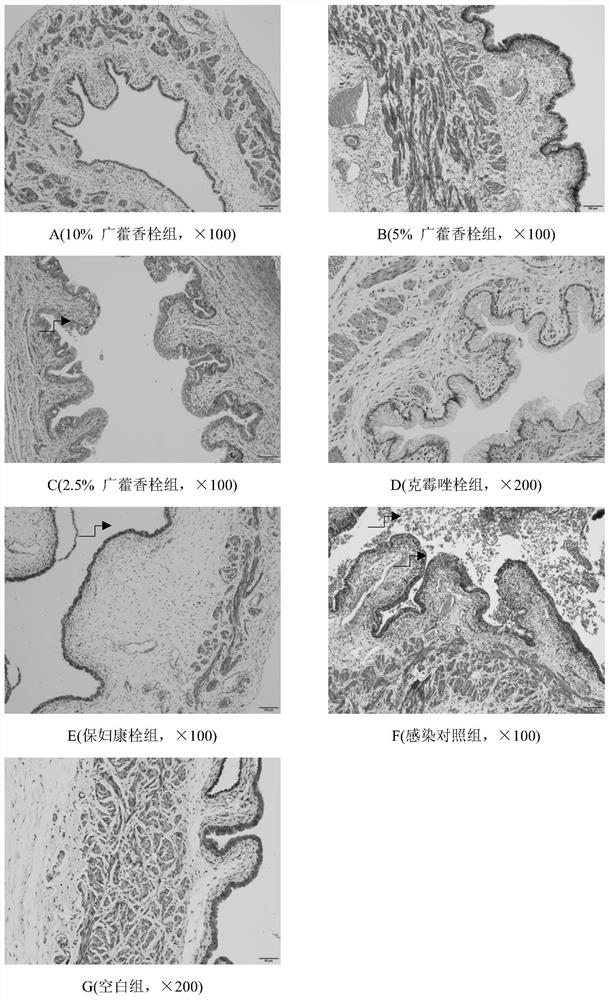
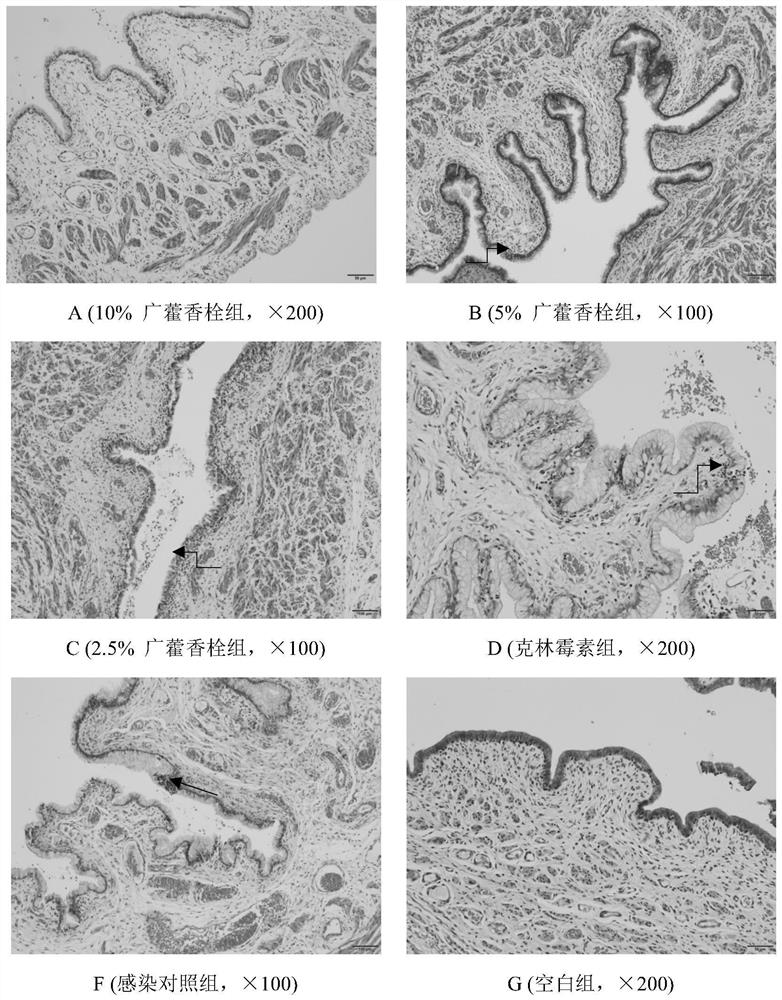
Pogostemon cablin suppository, preparation method and application
A technology of patchouli suppositories and patchouli, applied in suppository delivery, antifungal agents, pharmaceutical formulations, etc., can solve the problem that patchouli extract cannot fully exert vaginitis, achieve product quality control, and ensure clinical efficacy , Guaranteed curative effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Extraction and Content Determination of Patchouli Extract
[0032] The raw material is selected from patchouli decoction pieces that have not been crushed and infiltrated, and are extracted by water-distillation and steam method. The specific extraction method is as follows:
[0033] In step 1, the decoction pieces of patchouli are not crushed and infiltrated, and the extraction method is adopted by the water separation steam method, and the extraction time is 8 hours, and the extraction rate is the best.
[0034] In step 2, after the extraction is completed, the content of bucchiol (mg / g) and patchouli ketone (mg / g) in the extract are determined by gas chromatography.
[0035] The test results of the content of patchouli alcohol and patchouli ketone in different batches of patchouli extract are as follows,
[0036] Table 1 Determination of different batches of patchouli extract
[0037]
[0038] The patchouli oil contains not less than 30% of pothole alcohol, not...
Embodiment 2
[0040] 1) Prescription
[0041] The prescription composition of 1000 patchouli suppositories (2.0g / grain) is shown in Table 1, and the medicine content of patchouli suppository is 5%, and the ratio of auxiliary material is polyethylene glycol 4000: polyoxyl stearate (40) Esters: polyethylene glycol 400 = 6:3:4.
[0042] Table 2 The prescription composition of 1000 patchouli suppositories
[0043] Raw materials Mass (1000 capsules) Patchouli Extract 100g polyethylene glycol 4000 877g Polyoxyl(40) Stearate 438g polyethylene glycol 400 585g
[0044] 2) The preparation process is as follows
[0045] S1 Ingredients:
[0046] Weigh the ingredients according to the prescription, and take 5kg as the pilot batch.
[0047] S2 matrix melting
[0048] Take 2192g of PEG4000 (solid) and 1096g of polyoxyl (40) stearate (solid) into the emulsification tank in turn, set the lower limit of the temperature of the emulsification tank to 70°C, and the ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com