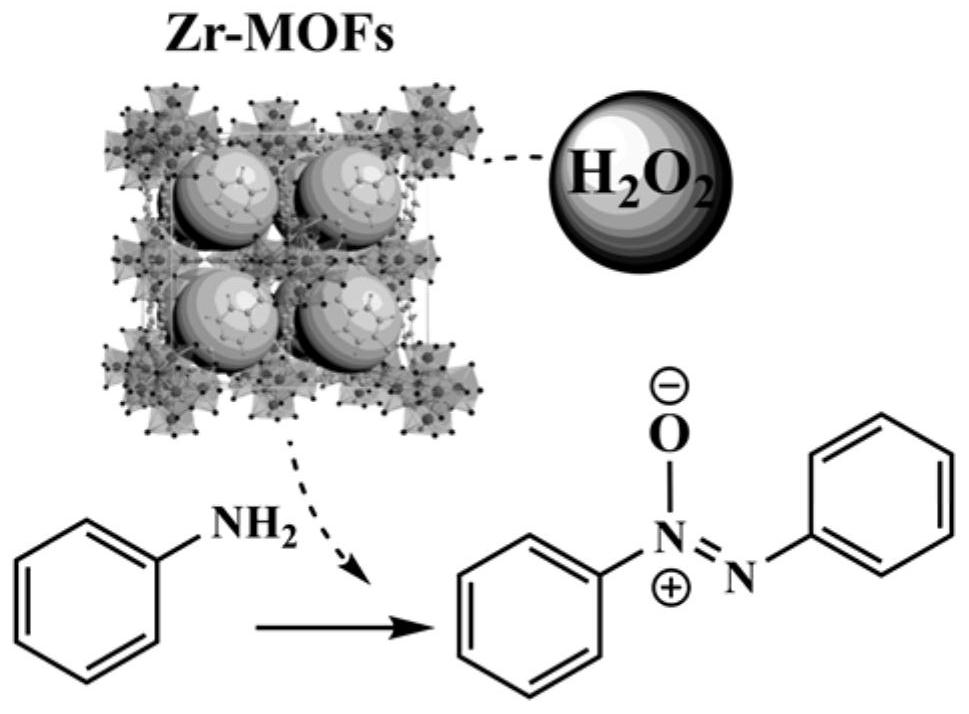
Method for preparing aromatic azoxycompound based on aromatic amine oxidation
A technology of azo oxide and aromatic amines, which is applied in chemical instruments and methods, organic compound/hydride/coordination complex catalysts, chemical/physical processes, etc., and can solve the problems of reaction equipment corrosion, alkaline wastewater easily polluting the environment, Does not meet the issues of green and sustainable development
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1-9
[0028] Example 1-9: Preparation of Zr-MOFs by hydrothermal method
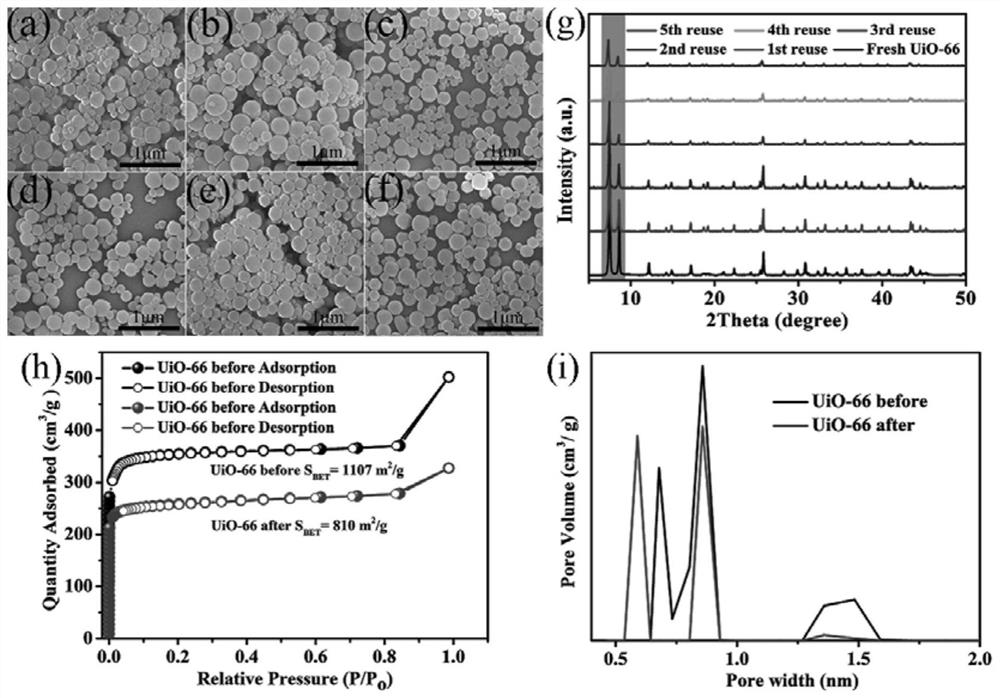
[0029] Taking UiO-66 as an example, 100mg (0.6mmol) terephthalic acid (H 2 BDC) and 140mg (0.6mmol) ZrCl 4Dissolve in 25mL DMF and add 1.03mL acetic acid with stirring. Sonicate the solution with an ultrasonic cleaner until clear. Subsequently, the solution was transferred to a 50 mL autoclave and heated at 120 °C for 24 h. After the autoclave was naturally cooled to room temperature, the product was centrifuged and washed at least three times with DMF and methanol, respectively. The white solid powder was collected after vacuum drying at 60°C. Other Zr-MOFs S The synthesis method is similar to that of UiO-66, as shown in Examples 1-9 in Table 1.
[0030] See Table 1 below for details.
[0031] Table 1. Zr-MOFs prepared by hydrothermal synthesis
[0032]
Embodiment 10-24
[0033] Embodiment 10-24: Reaction condition screening
[0034] Add 10mg of UiO-66 (Zr=2.67mmol%), 2mmol of aniline, 5mL of deionized water and 511μL of 30% hydrogen peroxide solution into the reaction tube, heat the oil bath to 60°C under magnetic stirring, and finish the reaction for 6h. The reaction product was analyzed by GC-MS, and the yield of azobenzene oxide was >99% (Example 16).
[0035] Except that reaction temperature, reaction time, catalyst consumption, hydrogen peroxide consumption are different, catalyst activity evaluation is identical with embodiment 1. The reaction conditions and catalytic reaction results are shown in Table 2. As can be seen from Table 2, reaction temperature, reaction time, catalyst consumption, hydrogen peroxide consumption have influence on catalytic effect. With reaction temperature (embodiment 10,13,16 and 17), reaction time, (embodiment 10,11 and 12 or 13,14 and 15), catalyst consumption (embodiment 18,19 and 20) and hydrogen peroxid...
Embodiment 25-33
[0039] Examples 25-33: Substrate Expansion
[0040] The evaluation of the catalyst activity was the same as in Example 10 except that the substrate was different. The results of the catalytic reactions are shown in Table 3. Aniline compounds with different substituents have yields of 40-99% aromatic azo compounds.
[0041] See Table 3 below for details.
[0042] Table 3 Different substrate expansion
[0043]
[0044]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com