Quantum dot joint detection test strip for influenza viruses A and B as well as preparation method and application of quantum dot joint detection test strip
A technology of influenza virus and quantum dots, which is applied in the field of medical testing, can solve the problems of related products that have not yet seen joint detection of influenza virus type A and type B antigens, and achieve the goal of ensuring stability, high sensitivity, and improving accuracy and sensitivity Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] Example 1 Influenza virus type A and type B antigen quantum dot joint detection test strip
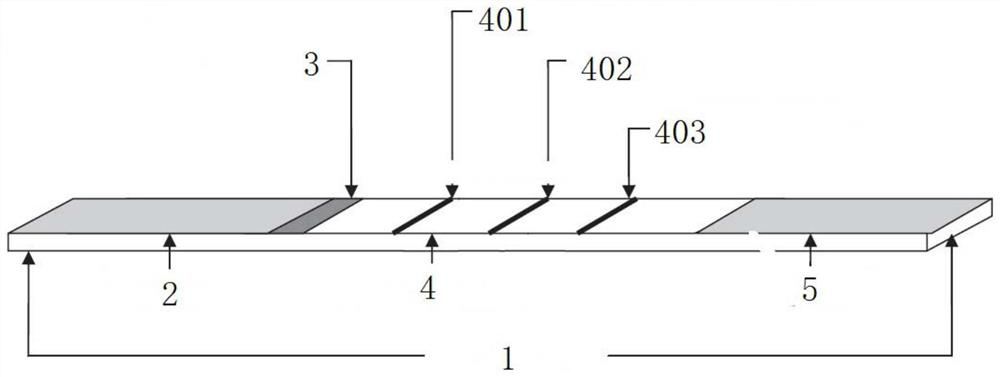
[0042] The test strip structure of this embodiment is as follows: figure 1 As shown, it includes a bottom plate 1, on which a sample pad 2, a binding pad 3, a nitrocellulose membrane 4, and a water-absorbing pad 5 are overlapped successively from left to right on the bottom plate, and the binding pad 3 is coated with quantum dot marks Influenza virus type A detection antibody and quantum dot-labeled influenza virus type B detection antibody; the nitrocellulose membrane is provided with a first detection line 401, a second detection line 402 and a quality control line 403, the first detection line 401 is coated with influenza virus type A capture monoclonal antibody, and the second detection line 402 is coated with influenza virus type B capture monoclonal antibody; the quality control line 403 is coated with rabbit anti-mouse IgG polyclonal antibody.
[0043] Wherein, in this e...
Embodiment 2
[0045] The preparation method of embodiment 2 test strips of the present invention
[0046] (1) Preparation of sample pad
[0047] Cut the sample pad into an appropriate size and length that matches the binding pad, spray the sample pad treatment solution on the sample pad at an amount of 50 μL / cm, soak it at room temperature for 1.5 hours, and dry it in a 37°C thermostat for no less than 15 hours Drying for use; The sample treatment solution is a phosphate buffer containing PVP10 (polyvinylpyrrolidone) 0.1wt%, polyethylene glycol-300 1.8wt%, TritonX-100 1.0wt% and 2-hydroxyethylamine 1.6wt% solution (0.015M, pH 7.2).
[0048] (2) Preparation of binding pads
[0049] ①Take 1.5 mg of carboxyl water-soluble quantum dots, and then suspend the quantum dots in 1200 μL of MES buffer solution with a concentration of 0.05M and a pH of 6.0;
[0050] ②Use 0.05M pH6.0 MES buffer to prepare NHS (50mg / ml) and EDC (50mg / ml), take 30μl each and add to quantum dots, the final concentration...
Embodiment 3
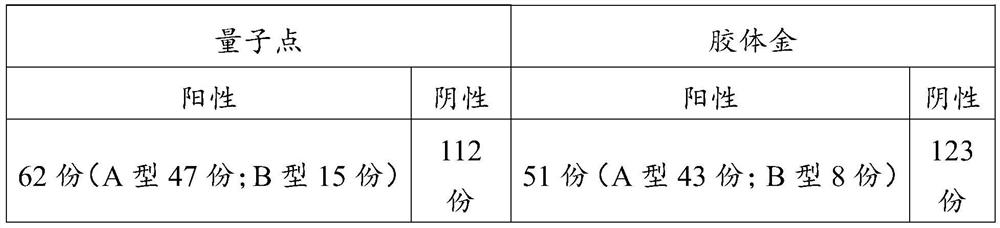
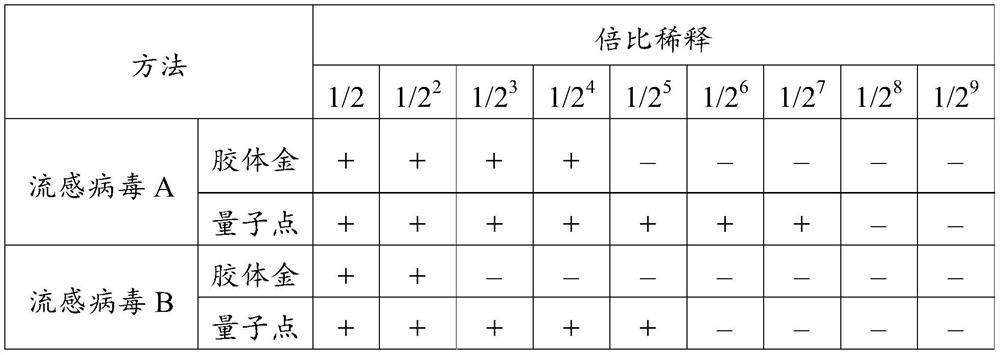
[0063] Example 3 Screening experiment of quantum dot complex solution
[0064] Use different quantum dot complex solutions to resuspend quantum dot-labeled influenza virus type A detection antibodies and quantum dot-labeled influenza virus type B detection antibodies, treatment 1: quantum dot complex solutions contain melezitose 2.5wt%, glycine 0.25 wt%, gentamicin sulfate 0.2wt%, cinnamaldehyde 0.12wt% and TritonX-100 0.1wt% phosphate buffer (0.01M, pH7.2); treatment 2: Quantum dot complex solution is containing aminoacetic acid 0.25 wt%, gentamycin sulfate 0.2wt%, cinnamaldehyde 0.12wt% and TritonX-100 0.1wt% phosphate buffer (0.01M, pH 7.2); treatment 3: Quantum dot complex solution containing melezitose 2.5 wt%, gentamicin sulfate 0.2wt%, cinnamaldehyde 0.12wt% and TritonX-100 0.1wt% in phosphate buffer (0.01M, pH 7.2). Each group of test strip samples was prepared according to the method described in Example 2 by using the above-mentioned different quantum dot complex so...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| length | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com