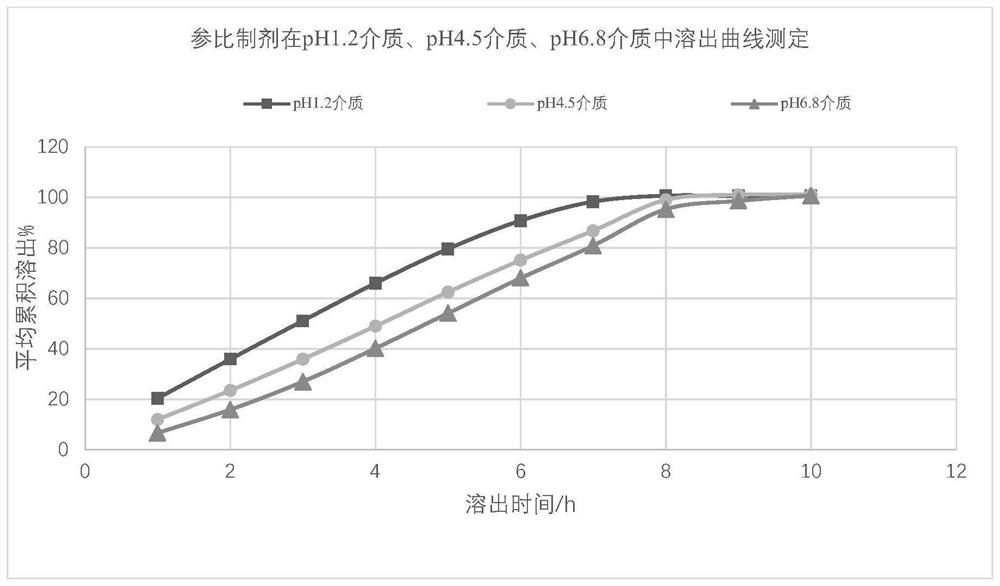
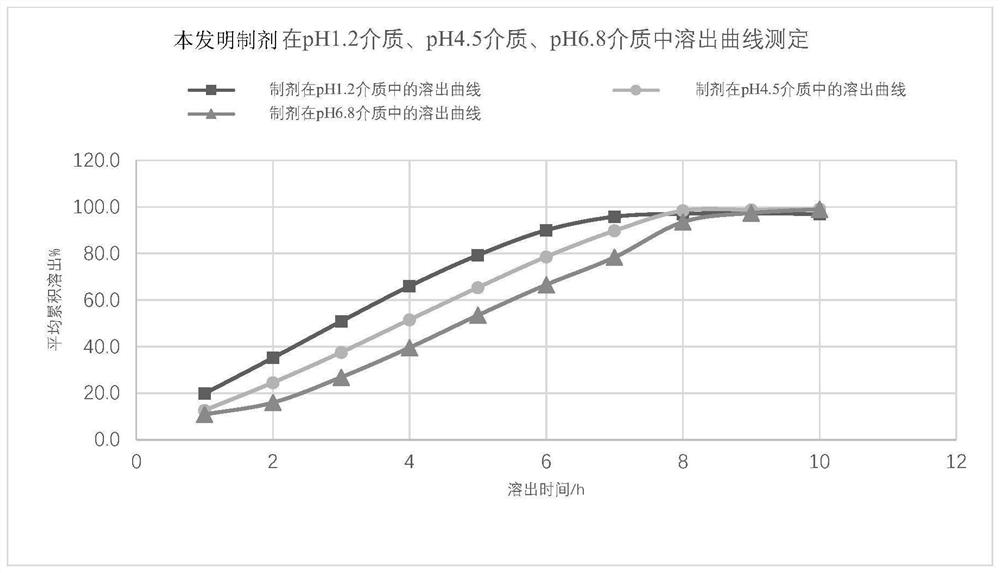
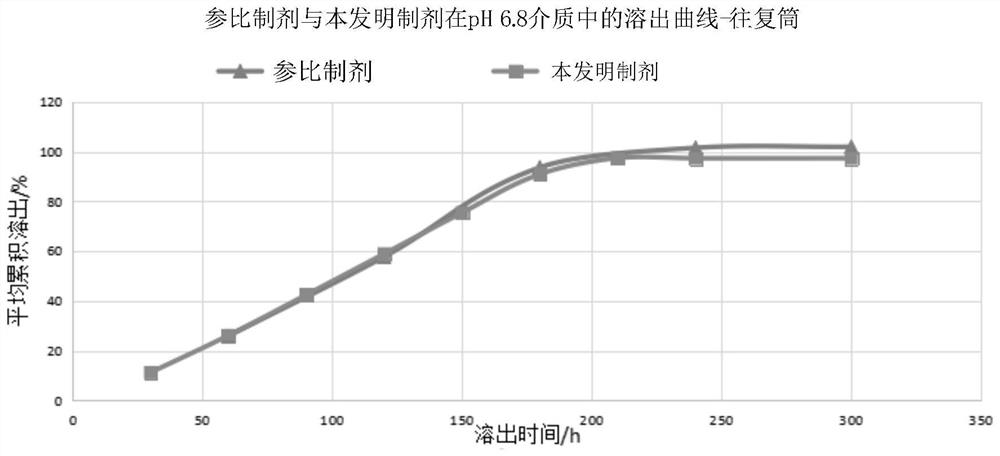
Mirabegron sustained release tablet as well as preparation method and quality detection method thereof
A technology for mirabegron and sustained-release tablets, applied in the field of medicine, can solve the problems of undetected female patients, excessive tablet weight differences, poor fluidity, etc., and achieve the effects of reducing potential safety hazards, long shelf life, and reducing production costs.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0088] The preparation of embodiment 1 Mirabegron sustained-release tablets
[0089] 1. Raw materials and dosage
[0090] The raw materials and dosages used to prepare Mirabegron sustained-release tablets in this example are shown in Table 1 below:
[0091] Table 1 Amount of raw and auxiliary materials
[0092]
[0093]
[0094] 2. Preparation method
[0095] (1) Take each raw and auxiliary material according to the percentage by weight described in Table 1;
[0096] (2) Premixing: Add Mirabegron, polyoxyethylene, polyethylene glycol and hydroxypropyl cellulose into the wet granulation pot, set the stirring blade at 250 rpm, the cutter at 600 rpm, and premix for 5 minutes.
[0097] (3) Liquid addition: set the rotation speed of the stirring blade to 170rpm, the rotation speed of the cutting knife to 1500rpm, and the atomization pressure to 0.0125MPa, add the wetting agent by spraying, and the liquid addition time is 2min.
[0098] (4) Granulation: set the stirring bl...
Embodiment 2
[0121] The preparation of embodiment 2 Mirabegron sustained-release tablets
[0122] 1. Raw materials and dosage
[0123] The raw materials and dosages used to prepare Mirabegron sustained-release tablets in this example are shown in Table 3 below:
[0124] Table 3 Amount of raw and auxiliary materials
[0125]
[0126] 2. Preparation method
[0127] (1) Take each raw and auxiliary material according to the percentage by weight described in Table 2;
[0128] (2) Premixing: Add mirabegron, polyoxyethylene, polyethylene glycol and hydroxypropyl cellulose into the wet granulation pot, set the stirring blade at 250 rpm, the cutting knife at 600 rpm, and premix for 5 to 10 minutes.
[0129] (3) Liquid addition: set the rotation speed of the stirring blade to 170rpm, the rotation speed of the cutting knife to 1500rpm, and the atomization pressure to 0.0125MPa, add the wetting agent by spraying, and the liquid addition time is 3min.
[0130] (4) Granulation: set the rotation s...
Embodiment 3
[0136] The preparation of embodiment 3 Mirabegron sustained-release tablets
[0137] 1. Raw materials and dosage
[0138] The raw materials and dosages used to prepare Mirabegron sustained-release tablets in this example are shown in Table 4 below:
[0139] Table 4 Amount of raw and auxiliary materials
[0140]
[0141] 2. Preparation method
[0142] (1) Take each raw and auxiliary material according to the percentage by weight described in Table 3;
[0143] (2) Premixing: Add Mirabegron, polyoxyethylene, polyethylene glycol and hydroxypropyl cellulose into the wet granulation pot, set the stirring blade at 150 rpm, the cutter at 600 rpm, and premix for 10 minutes.
[0144] (3) Liquid addition: set the rotation speed of the stirring blade to 150rpm, the rotation speed of the cutting knife to 1500rpm, and the atomization pressure to 0.0125MPa, add the wetting agent by spraying, and the liquid addition time is 1min.
[0145] (4) Granulation: set the rotation speed of the ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| hardness | aaaaa | aaaaa |
| wavelength | aaaaa | aaaaa |
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com