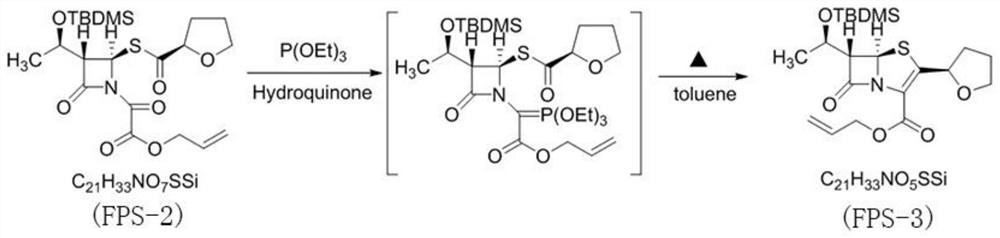
Preparation method of faropenem sodium
A technology of faropenem sodium and ethyl, applied in the field of medicinal chemistry, can solve the problems of low yield, many preparation steps, influence on the yield of faropenem sodium, etc., and achieve the effects of high yield and high purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
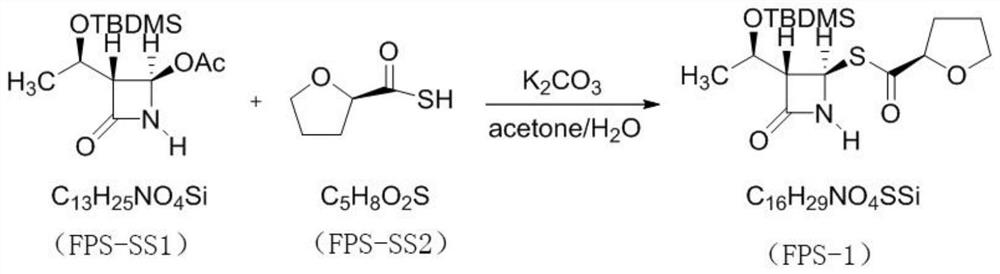
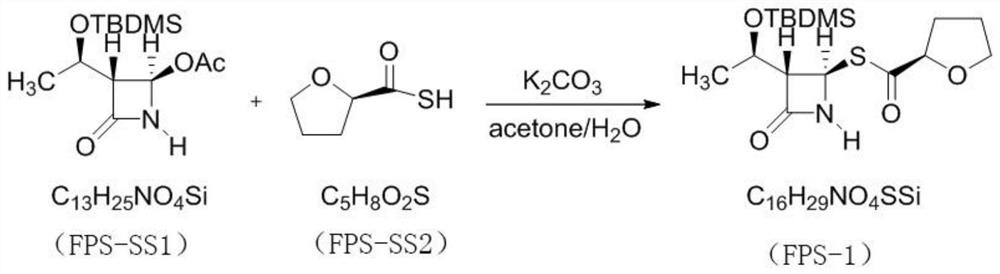
[0027] The invention provides a preparation method of faropenem sodium, comprising the following steps:
[0028] S1. Potassium carbonate, R-(+)-thiotetrahydrofuran-2-carboxylic acid, (3R,4R)-4-acetoxy-3-[(R)-(tert-butyldimethylsilyloxy) Ethyl]azetidin-2-one is mixed, and after the reaction, (3S,4R)-3-[(1R)-(tert-butyldimethylsilyloxy)ethyl]-4-[(2R) -tetrahydrofuroylthio]azetidin-2-one. Among them, the potassium carbonate, R-(+)-thiotetrahydrofuran-2-carboxylic acid, (3R,4R)-4-acetoxy-3-[(R)-(tert-butyldimethylsilyloxy ) ethyl] azetidin-2-one in a molar ratio of 1:1:1.
[0029] For ease of writing and reading, (3R,4R)-4-acetoxy-3-[(R)-(tert-butyldimethylsilyloxy)ethyl]azetidin-2-one is denoted as FPS-SS1, R-(+)-thiotetrahydrofuran-2-carboxylic acid is recorded as FPS-SS2, and (3S,4R)-3-[(1R)-(tert-butyldimethylsilyloxy)ethyl Base]-4-[(2R)-tetrahydrofuroylthio]azetidin-2-one is designated as FPS-1.
[0030] specifically,
[0031] Dissolve potassium carbonate in water, add ...
Embodiment 1
[0057] This embodiment provides a preparation method of faropenem sodium, comprising the following steps:
[0058] Synthesis of S1, FPS-1
[0059] Dissolve 1.2 mol of anhydrous potassium carbonate in 287.43 g of purified water, add 1.2 mol of FPS-SS2 dropwise at 10-20°C, after the addition is complete, stir and react for 30-60 minutes to obtain a solution;
[0060] Dissolve 287.43g (1mol) FPS-SS1 in 287.43g acetone to obtain a second solution;
[0061] Slowly add the B solution to the A solution, rinse the container holding the B solution and the instrument for transferring the B solution with 287.43 g of acetone, add it to the above reaction solution, and react at 30-35°C for 4-6 hours;
[0062] After the TLC detection reaction was completed, 287.43g of toluene was added for extraction, and the organic phase was collected;
[0063] The organic phase is backwashed twice with purified water (the purified water used for backwashing is 287.43g each time here), after washing, th...
Embodiment 2
[0089] This embodiment provides a preparation method of faropenem sodium, comprising the following steps:
[0090] Synthesis of S1, FPS-1
[0091] Dissolve 1.2 mol of anhydrous potassium carbonate in 287.43 g of purified water, add 1.2 mol of FPS-SS2 dropwise at 15°C, after the addition is complete, stir and react for 45 minutes to obtain a solution;
[0092] Dissolve 287.43g (1mol) FPS-SS1 in 287.43g acetone to obtain a second solution;
[0093] Slowly add the B solution to the A solution, rinse the container holding the B solution and the instrument for transferring the B solution with 287.43 g of acetone, add it to the above reaction solution, and react at 33°C for 5 hours;
[0094] After the TLC detection reaction was completed, 287.43g of toluene was added for extraction, and the organic phase was collected;
[0095] The organic phase is backwashed twice with purified water (the purified water used for backwashing is 287.43g each time here), after washing, the organic p...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com