Non-noble metal catalyst for fuel cell and preparation method of non-noble metal catalyst
A fuel cell, non-precious metal technology, applied in battery electrodes, circuits, electrical components, etc., can solve problems such as the gap between ORR activity and catalytic stability, difficult to control reaction conditions, and complicated preparation process steps for non-precious metal catalysts, achieving easy industrialization. The effect of production promotion, simple synthesis steps and strong controllability of reaction parameters
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
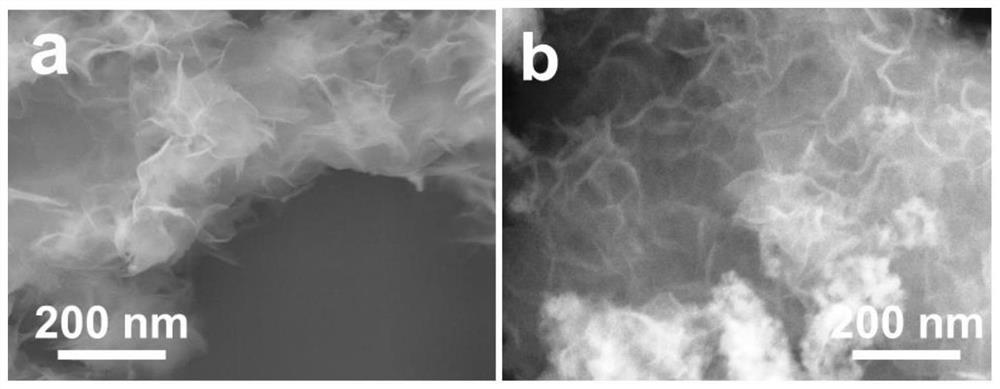
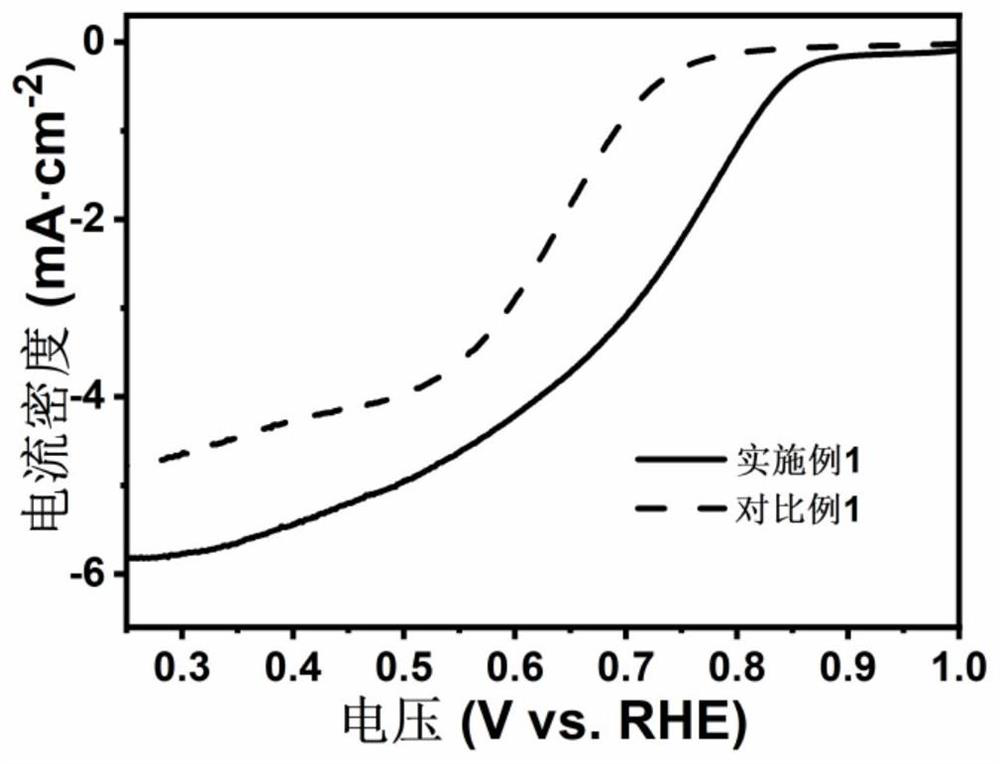
[0022] Weigh 5mmol of cobalt chloride, 2.5mmol of potassium cobaltcyanide and 3g of graphene oxide, dissolve them in 5mL of deionized water, and obtain a black hydrogel after standing in an oven at 50°C for 10 hours; prepare 1g / mL of NaBH 4 Solution 100mL, poured into the above hydrogel and stirred at 80°C for 6h to obtain graphene oxide-supported cobalt tetraoxide nanosheets; disperse the obtained graphene oxide-supported cobalt tetraoxide nanosheets in ethanol and water to obtain an electrocatalyst dispersion , used as a fuel cell catalyst.
Embodiment 2
[0024] Weigh 10mmol of cobalt chloride, 5mmol of potassium cobaltcyanide and 4g of graphene oxide and dissolve them in 10mL of deionized water. After standing in an oven at 50°C for 8 hours, a black hydrogel is obtained; prepare 2g / mL of NaBH 4 Solution 100mL, poured into the above hydrogel and stirred at 80°C for 10h to obtain graphene oxide-supported cobalt tetraoxide nanosheets; disperse the obtained graphene oxide-supported cobalt tetraoxide nanosheets in ethanol and water to obtain an electrocatalyst dispersion , used as a fuel cell catalyst.
Embodiment 3
[0026] Weigh 3mmol of cobalt chloride, 1.5mmol of potassium cobaltcyanide and 2g of graphene oxide, dissolve them in 3mL of deionized water, and obtain a black hydrogel after standing in an oven at 50°C for 12 hours; prepare 1g / mL of NaBH 4 80 mL of the solution was poured into the above hydrogel and stirred at 80°C for 8 hours to obtain graphene oxide-supported cobalt tetraoxide nanosheets; the obtained graphene oxide-supported cobalt tetraoxide nanosheets were dispersed in ethanol and water to obtain an electrocatalyst dispersion , used as a fuel cell catalyst.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com