Chitosan oligosaccharide enteric-coated dropping pill and application thereof in preparation of medicine for treating non-alcoholic fatty liver disease
A technology of chitosan oligosaccharides and soluble drop pills, which is applied in the field of medicine, can solve the problems of lack of research on the activity and mechanism of chitosan oligosaccharides, easy oxidation and hydrolysis of chitosan oligosaccharides, and poor roundness of enteric-coated dripping pills. To achieve the effects of improving abnormal liver function indicators, enhancing serum antioxidant function, and avoiding oxidation and hydrolysis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
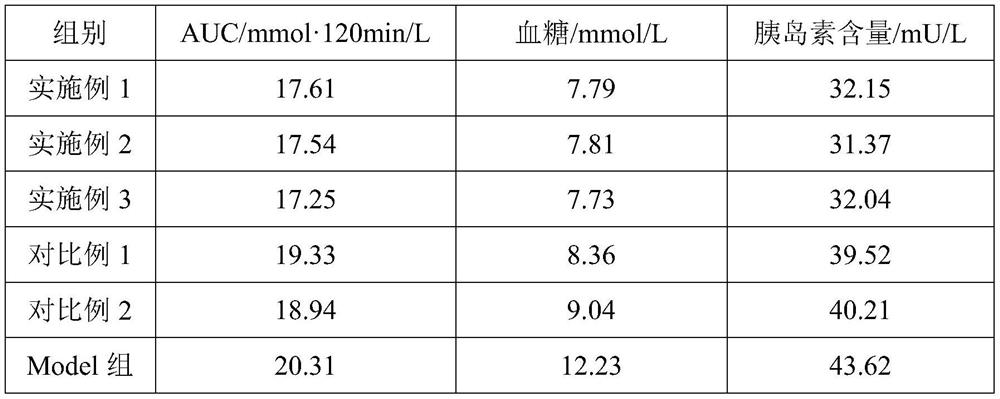
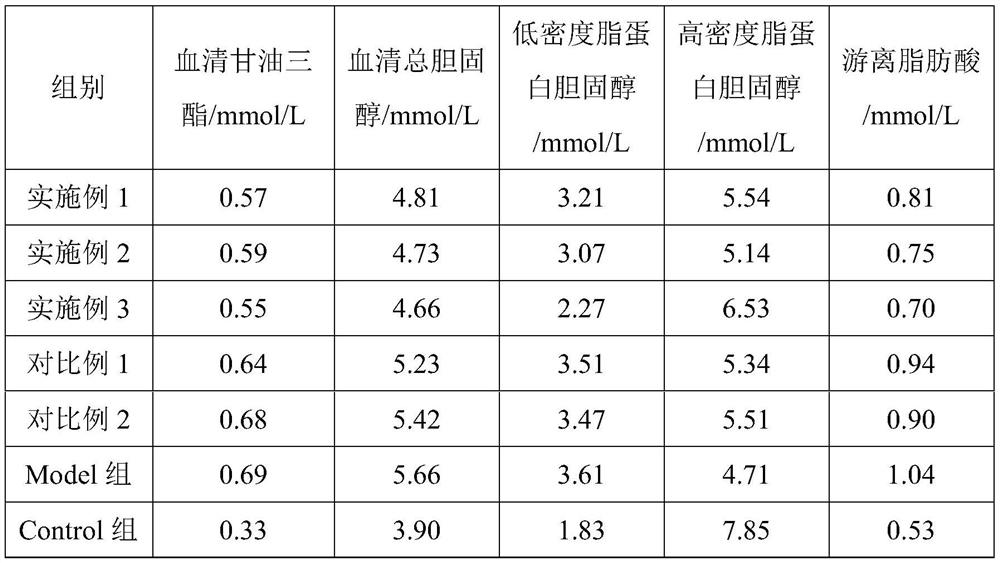
Embodiment 1
[0028] Embodiment 1, a kind of chitosan oligosaccharide enteric-coated dripping pill and preparation method thereof
[0029] The chitosan oligosaccharide enteric-coated dripping pill is composed of an enteric-coated layer coating and a ball core at a mass ratio of 6:13.
[0030] The enteric layer coating in the chitosan oligosaccharide enteric-coated dropping pills consists of the following components and parts by weight: 20 parts of Eudragit L100-55, 10 parts of talcum powder, 2 parts of polyethylene glycol 6000, ethanol 218 copies.
[0031] The preparation method of the enteric layer coating in the chitosan oligosaccharide enteric-coated dropping pills is as follows: take the Eudragit L100-55 of formula quantity, slowly add to 95 % ethanol, prepared into a suspension with a mass fraction of L100-55 of 8%, and passed through a 80-mesh sieve.
[0032] The core of the oligochitosan enteric-coated dripping pill is composed of the following components and parts by weight thereo...
Embodiment 2
[0035] Embodiment 2, a kind of chitosan oligosaccharide enteric-coated dripping pill and preparation method thereof
[0036] The chitosan oligosaccharide enteric-coated dropping pill is composed of an enteric-coated layer coating and a ball core in a mass ratio of 10:17.
[0037] The enteric layer coating in the chitosan oligosaccharide enteric-coated dropping pills consists of the following components and parts by weight: 40 parts of Eudragit L100-55, 20 parts of talcum powder, 4 parts of polyethylene glycol 6000, ethanol 436 copies.
[0038] The preparation method of the enteric layer coating in the chitosan oligosaccharide enteric-coated dropping pills is as follows: take the Eudragit L100-55 of formula quantity, slowly add to 95 % ethanol, prepared into a suspension with a mass fraction of L100-55 of 8%, passed through an 80-mesh sieve, and obtained.
[0039] The core of the oligochitosan enteric-coated dripping pill is composed of the following components and parts by w...
Embodiment 3
[0042] Embodiment 3, a kind of chitosan oligosaccharide enteric-coated dripping pill and preparation method thereof
[0043] The chitosan oligosaccharide enteric-coated dripping pill is composed of an enteric-coated layer coating and a ball core at a mass ratio of 8:15.
[0044] The enteric layer coating in the chitosan oligosaccharide enteric-coated dropping pills consists of the following components and parts by weight: 31 parts of Eudragit L100-55, 16 parts of talcum powder, 3 parts of polyethylene glycol 6000, ethanol 326 copies.
[0045] The preparation method of the enteric layer coating in the chitosan oligosaccharide enteric-coated dropping pills is as follows: take the Eudragit L100-55 of formula quantity, slowly add to 95 % ethanol, prepared into a suspension with a mass fraction of L100-55 of 8%, passed through an 80-mesh sieve, and obtained.
[0046] The core of the oligochitosan enteric-coated dripping pill is composed of the following components and parts by we...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com