Method for photocatalytic reduction cross-coupling of aromatic olefin and halogenated hydrocarbon
A cross-coupling and aromatic technology, applied in the field of photocatalytic organic synthesis, can solve the problems that have not yet been reported, and achieve good yield, high photocatalytic conversion efficiency, and simple and easy-to-operate reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022]
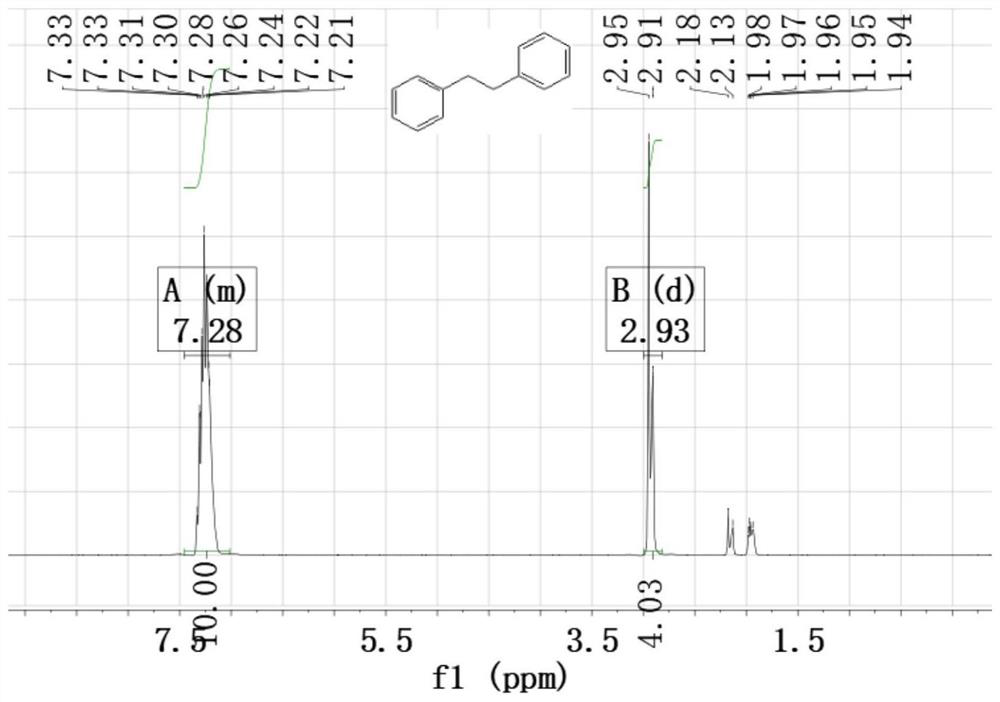
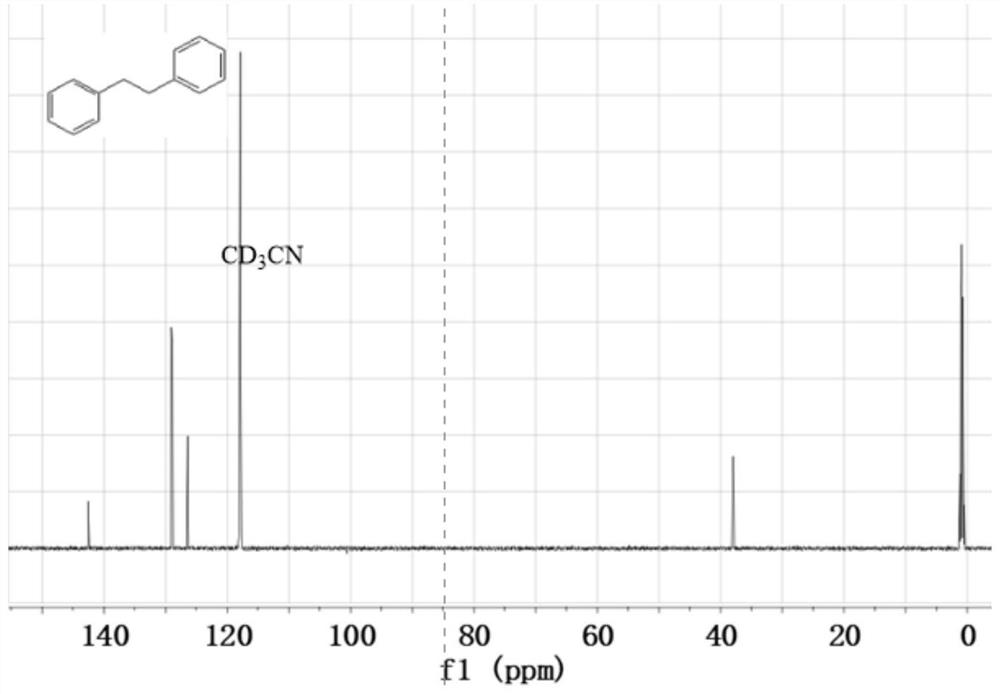
[0023] Add 10 mg of 15% Cu with an average particle size of 4 nm to the pyrex tube 2+ -MPA-CdS photocatalyst, 115μL (1mmol) styrene, 105μL (1mmol) bromobenzene, 224mg (4mmol) potassium hydroxide, 5mL methanol and deionized water volume ratio of 1:1 mixed solvent, mix well, make the system In a sealed nitrogen atmosphere, irradiate with a 420nm LED lamp for 12 hours to obtain 1,2-diphenylethane with a yield of 62%, and the structural characterization data are: 1 H NMR (400MHz, CDCl 3 ): δ7.34-7.24(m,4H), 7.20(dd,6H), 2.92(s,4H), see figure 1 ; 13 C NMR (400MHz, CDCl 3 ): δ141.8, 128.4, 128.3, 125.9, 37.9, see figure 2 .
Embodiment 2
[0025]
[0026] In this example, the styrene in Example 1 was replaced with 133 μL (1 mmol) 4-methoxystyrene, and the other steps were the same as in Example 1 to obtain 1-methoxy-4-(2-phenylethyl) Benzene, its yield is 87%, and the structural characterization data is: 1 H NMR (400MHz, CDCl 3 ):δ7.34-7.28(m,2H),7.22(ddd,,3H),7.15-7.07(m,2H),6.89-6.82(m,2H),3.81(s,3H),2.91(d, 4H); 13 C NMR (400MHz, CDCl 3 ): δ157.8, 141.8, 133.9, 129.3, 128.4, 128.3, 125.8, 113.7, 55.2, 38.2, 37.0.
Embodiment 3
[0028]
[0029] In this example, 131 μL (1 mmol) of 4-chlorostyrene was used to replace the styrene in Example 1, and the other steps were the same as in Example 1 to obtain 1-chloro-4-(2-phenylethyl)benzene, which produced The ratio is 40%, and the structural characterization data are: 1 H NMR (400MHz, CDCl 3 ):δ7.30-7.17(m,5H),7.16-7.11(m,2H),7.10-7.03(m,2H),2.88(s,4H); 13 C NMR (400MHz, CDCl 3 ): δ141.2, 140.1, 131.6, 129.8, 128.4, 128.4, 128.3, 126.0, 37.7, 37.2.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com