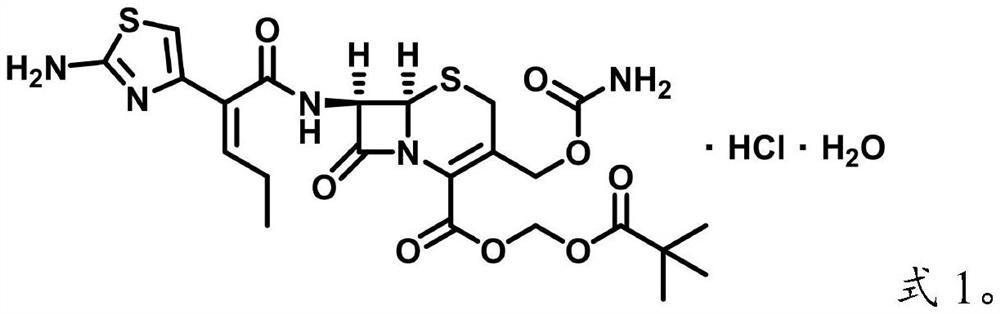
Preparation method of E-type isomer of cefcapene pivoxil
A technology for cefcapine pivoxil and isomers, which is applied in the field of preparation of cefcapine pivoxil E isomers, and can solve the problems of no introduction of the synthesis method of cefcapine pivoxil E isomers and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
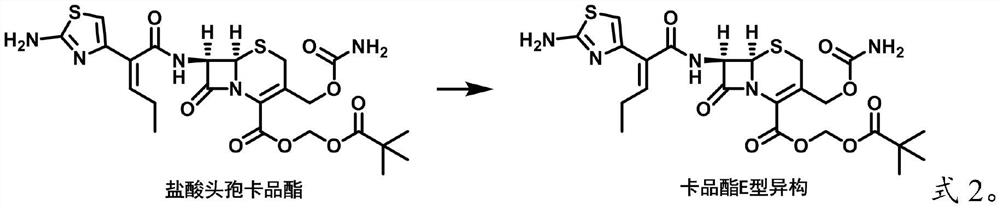
[0022] The invention provides a kind of preparation method of cefcapene pivoxil E-type isomer, comprising the following steps:
[0023] The organic solution of cefcapene pivoxil raw material is heated to reflux, obtains cefcapene pivoxil E-type isomer, and described cefcapene pivoxil raw material comprises the inorganic salt of cefcapene pivoxil and / or cefcapene pivoxil, and described heating reflux The insulation temperature is ≥40°C.
[0024] In the present invention, unless otherwise specified, the raw materials used are commercially available products well known to those skilled in the art.
[0025] In the present invention, the organic solution of the raw material of cefcapene pivoxil preferably includes the raw material of cefcapene axetil and an organic solvent (hereinafter referred to as the first organic solvent).
[0026] In the present invention, the raw material of cefcapene pivoxil preferably includes an inorganic salt of cefcapene pivoxil.
[0027] In a specifi...
Embodiment 1
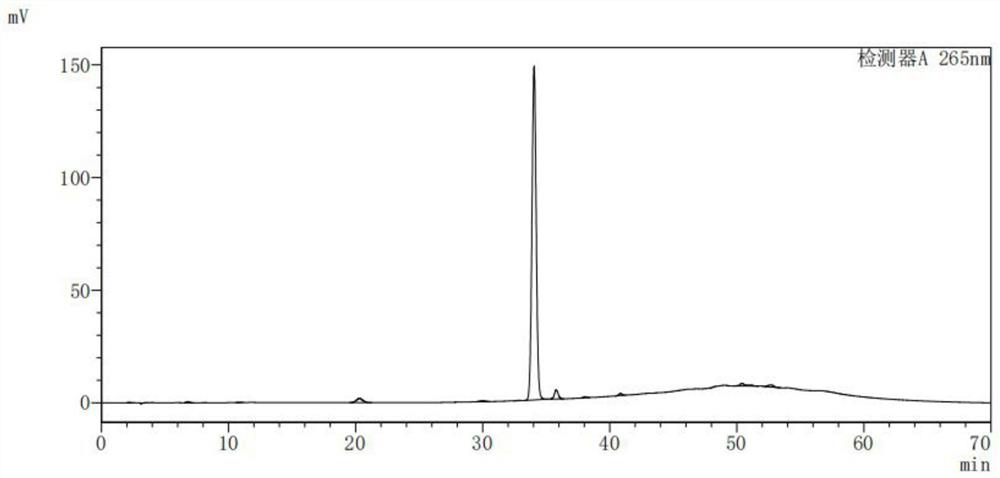
[0069] At 20°C, add 10g of cefcapene pivoxil hydrochloride and 100mL of methanol into a 500mL flask, stir until the system dissolves, raise the temperature in a water bath to 50°C, stir for 3-4 hours, and monitor cefcapene pivoxil E by thin-layer chromatography and HPLC. The reaction can be stopped when the isomer content does not increase. Concentrate the reaction solution under reduced pressure at 40°C to a yellow solid. Dissolve the yellow solid in 100mL of ethyl acetate and stir evenly. Then add 50mL of pure water and add 10% Adjust the sodium hydroxide solution to pH = 6, stir for 30 minutes, separate layers, dry over anhydrous sodium sulfate, filter, and concentrate to dryness under reduced pressure at 30-45°C to obtain the crude product of cefcapine isomer type E; wet-packed silica gel Column, separate and purify the sample with the mobile phase of dichloromethane:methanol=35:1 (v:v), concentrate under reduced pressure, and dry the material with 30°C to obtain 6.0g of dr...
Embodiment 2
[0071] At 20°C, add 10g of cefcapene axetil hydrochloride and 90mL of methanol into a 500mL flask, stir until the system dissolves, raise the temperature in a water bath to 50°C, stir for 3-4 hours, and monitor cefcapene axetil E by thin-layer chromatography and HPLC. The reaction can be stopped when the isomer content does not increase. Concentrate the reaction solution under reduced pressure at 40°C to a yellow solid. Dissolve the yellow solid in 90mL of dichloromethane and stir evenly. Then add 50mL of pure water and add 10% Adjust the sodium hydroxide solution to pH = 6, stir for 30 minutes, separate layers, dry over anhydrous sodium sulfate, filter, and concentrate to dryness under reduced pressure at 30-45°C to obtain the crude product of cefcapine isomer type E; wet-packed silica gel Column, separate and purify the sample with the mobile phase of dichloromethane:methanol=40:1 (v:v), concentrate under reduced pressure, and dry the material with 30°C to obtain 5.8g of dry ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com