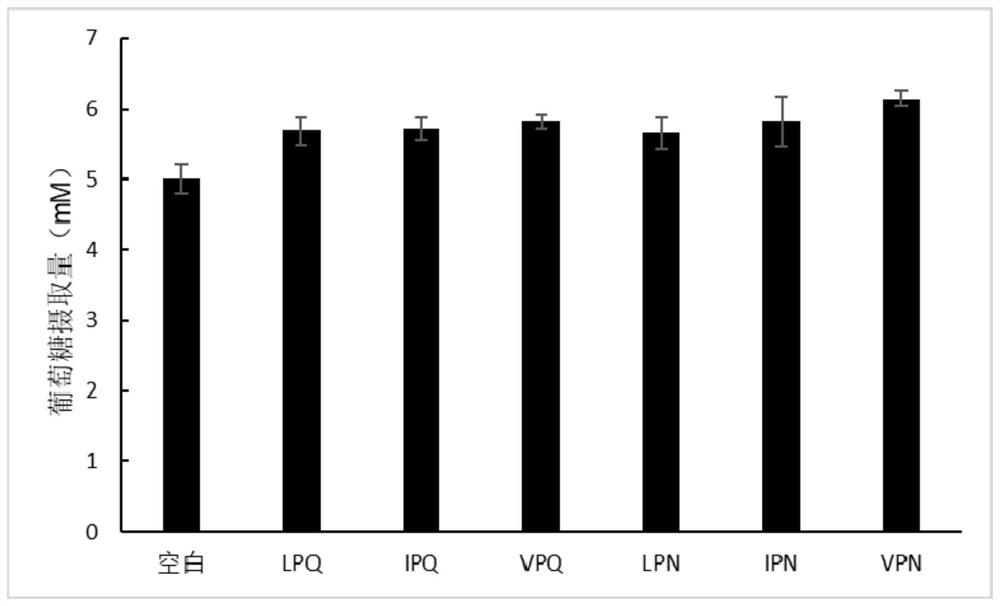
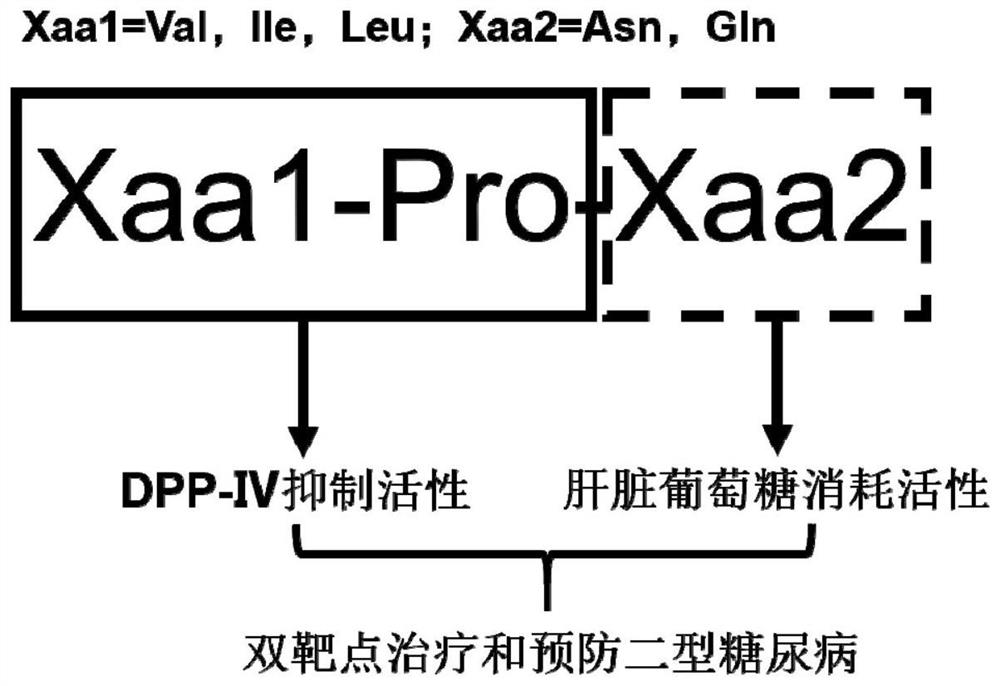
Tripeptide with double-target hypoglycemic function and application thereof
A dual-target, hypoglycemic technology, applied in tripeptide components, peptides, metabolic diseases, etc., can solve the problems of poor patient compliance and increase costs, and achieve the effects of reducing hyperglycemia, inhibiting degradation, and promoting insulin secretion.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
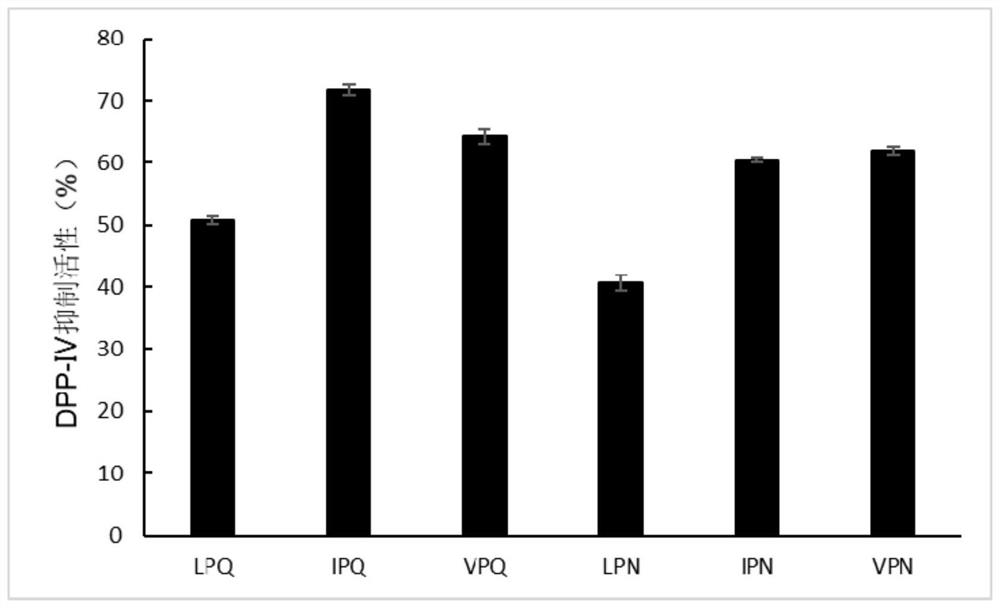
[0028] Example 1: DPP-IV inhibitory activity of tripeptide 1 (its sequence is as shown in SEQ ID NO.1 IPQ) with dual target hypoglycemic function
[0029] The DPP-IV inhibitory activity assay method is as follows: In a 96-well plate, add 80 μL of sample (tripeptide 1 with dual-target hypoglycemic function, sequence shown in SEQ ID NO.1) and 80 μL of 0.5 mM substrate Gly-Pro -pNA, mix well, place in a microplate reader and incubate at 37°C for 10min, add 40μL of DPP-IV enzyme solution with a concentration of 12.5mU / mL (preheated at 37°C for 3min), shake for 30s, and read at 405nm, Read every 2 minutes for a total of 120 minutes to monitor the release of pNA. The same volume of 100mM pH 8.0 Tris-HCl buffer was used as the control group instead of the sample. The above reagents or samples are prepared or diluted with the Tris-HCl buffer solution. Plot the absorbance value (as the ordinate) versus time (as the abscissa), in the linear range (R 2 >0.995) to obtain the slope of t...
Embodiment 2
[0032] Example 2: DPP-IV inhibitory activity of tripeptide 2 (its sequence is as shown in SEQ ID NO.2 LPQ) with dual-target hypoglycemic function
[0033] The DPP-IV inhibitory activity assay method is as follows: In a 96-well plate, add 80 μL of sample (tripeptide 2 with dual-target hypoglycemic function, sequence shown in SEQ ID NO.2) and 80 μL of 0.5 mM substrate Gly-Pro -pNA, mix well, place in a microplate reader and incubate at 37°C for 10min, add 40μL of 12.5mU / mL DPP-IV enzyme solution (preheated at 37°C for 3min), shake for 30s, and read at 405nm, every Read once every 2 minutes for a total of 120 minutes to monitor the release of pNA. The same volume of 100mM pH 8.0 Tris-HCl buffer was used as the control group instead of the sample. The above reagents or samples are prepared or diluted with the Tris-HCl buffer solution. Plot the absorbance value (as the ordinate) versus time (as the abscissa), in the linear range (R 2 >0.995) to obtain the slope of the curve, and...
Embodiment 3
[0036] Example 3: DPP-IV inhibitory activity of tripeptide 3 (its sequence is VPQ as shown in SEQ ID NO.3) with dual-target hypoglycemic function
[0037] The DPP-IV inhibitory activity assay method is as follows: In a 96-well plate, add 80 μL of sample (tripeptide 3 with dual-target hypoglycemic function, sequence shown in SEQ ID NO.3) and 80 μL of 0.5 mM substrate Gly-Pro -pNA, mix well, place in a microplate reader and incubate at 37°C for 10min, add 40μL of 12.5mU / mL DPP-IV enzyme solution (preheated at 37°C for 3min), shake for 30s, and read at 405nm, every Read once every 2 minutes for a total of 120 minutes to monitor the release of pNA. The same volume of 100mM pH 8.0 Tris-HCl buffer was used as the control group instead of the sample. The above reagents or samples are prepared or diluted with the Tris-HCl buffer solution. Plot the absorbance value (as the ordinate) versus time (as the abscissa), in the linear range (R 2 >0.995) to obtain the slope of the curve, and...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com