Preparation method and application of drug-loaded slow-release spider silk protein membrane
A technology of spidroin and drug loading, which can be used in pharmaceutical formulations, medical preparations containing active ingredients, drug combinations, etc. It can solve the problems of rare film-forming materials, reduce pain and distress, and achieve biocompatibility Good, improve the effect of drug administration
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] Example 1: Preparation of drug-loaded material matrix spidroin film
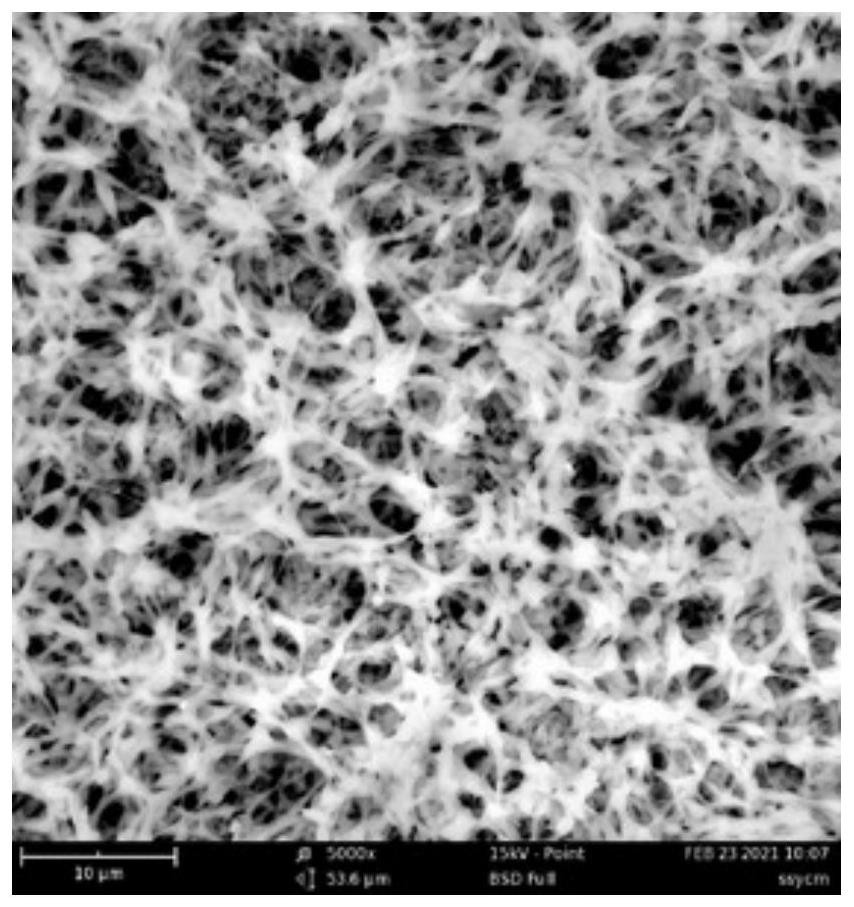
[0041] This embodiment provides a method for preparing a drug-loaded material matrix spidroin film, that is, a blank film that does not contain any drug. The Jingzhao tasseled spider silk used in the experiment came from Hainan Spider King Biotechnology Co., Ltd.
[0042] 1.1 Preparation of the drug-loaded material matrix spidroin protein solution: take the degummed Jingzhao tasseled spider silk to pick and remove impurities, weigh 100.0mg of the spider silk raw material and dissolve it in 600mL of hexafluoroisopropanol (HFIP) solution, and magnetically stir Heat to 50°C and reflux for 12 hours to dissolve; filter the dissolved spidroin protein solution while hot with a 400-mesh stainless steel metal filter to obtain a pure drug-loaded material matrix spidroin filtrate.
[0043] 1.2 Preparation of drug-loaded material matrix spidroin film: Spread the spidroin filtrate prepared under item 1.1 in a fla...
Embodiment 2
[0044] Example 2: Preparation of drug-loaded spidroin protein rehabilitation drug extract film
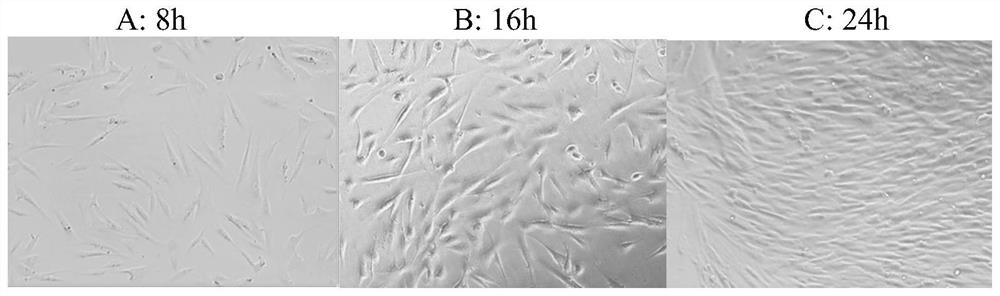
[0045] This embodiment provides a preparation method of drug-loaded spidroin protein rehabilitation drug extract film. The Jingzhao tasseled spider silk used in the test comes from Hainan Spider King Biotechnology Co., Ltd. The new rehabilitation drug in this example refers to the new rehabilitation drug extract (that is, the raw material extract of Kangfu new liquid) produced by Sichuan Good Doctor Panxi Pharmaceutical Co., Ltd. Limited offer.
[0046] 2.1 Preparation of drug-loaded material matrix spidroin protein solution: take degummed Jingzhao tasseled spider silk, sort and remove impurities, weigh 500.0 mg of spider silk raw material and place it in a 2000 mL round bottom flask, measure 1500 mL of hexafluoroiso Pour the propanol solution into a round-bottomed flask, heat it to 60°C with magnetic stirring and reflux for 10 hours to dissolve it; filter the dissolved spidroin s...
Embodiment 3
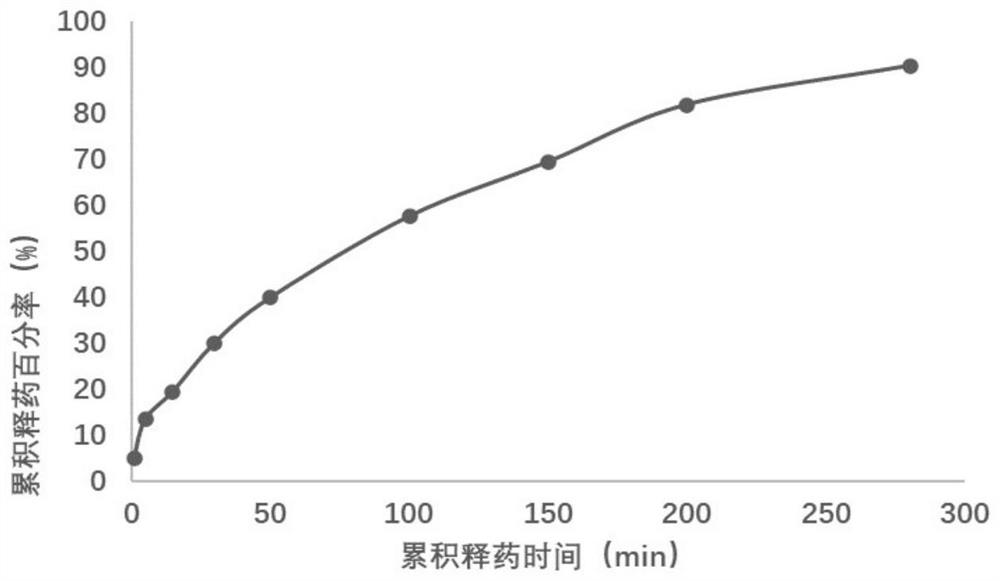
[0049] Example 3: Preparation of Strontium Chloride Membrane of Drug-Loaded Spidroin Protein Kangfuxin Drug Extract
[0050] This embodiment provides a method for preparing a strontium chloride (desensitizing agent) film of drug-loaded spidroin protein rehabilitation drug extract. The Jingzhao tasseled spider silk used in the test comes from Hainan Spider King Biotechnology Co., Ltd. In this example, the new rehabilitation drug refers to the raw material drug Kangfuxin drug extract (that is, the raw material extract of Kangfuxin solution) from Sichuan Provided by Good Doctor Panxi Pharmaceutical Co., Ltd.; strontium chloride was purchased from Shanghai Yuanye Biotechnology Co., Ltd.
[0051] 3.1 Preparation of drug-loaded material matrix spidroin protein solution: take degummed Jingzhao tasseled spider silk, sort and remove impurities, weigh 500.0mg of spider silk raw material into a 5000mL round bottom flask, measure 4000mL of hexafluoroisopropyl Pour the alcohol solution in...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com