Method for improving tolerance of corynebacterium glutamicum to inhibitors
A Corynebacterium glutamicum, inhibitor technology, applied in the field of bioengineering, can solve problems such as bacterial growth stress
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
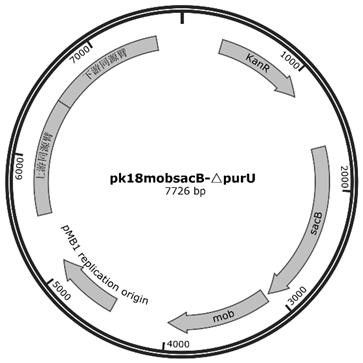
[0035] Example 1 Knockout purU Genetic construction of recombinant Corynebacterium glutamicum
[0036] (1) with C. glutamicum The genomic DNA of ATCC13032 is used as a template, and it is designed to be knocked out according to the sequence of SEQ ID NO.3 purU The left and right homologous arms of the gene (about 1kb each) and their primer sequences were amplified by PCR respectively, and the products were purified and recovered, and then combined with enzyme-digested pK18 mobsacB The plasmid fragments are connected by homologous recombination of three fragments, and the connection products are introduced by chemical transformation E. coli DH5α competent cells were spread onto LB solid plates added with Kan resistance, and cultured overnight in a 37°C incubator. After the monoclonal grows, pick the monoclonal and inoculate it into the LB liquid medium added with Kan resistance, extract the recombinant plasmid from the bacterial solution, and obtain the recombinant pla...
Embodiment 2
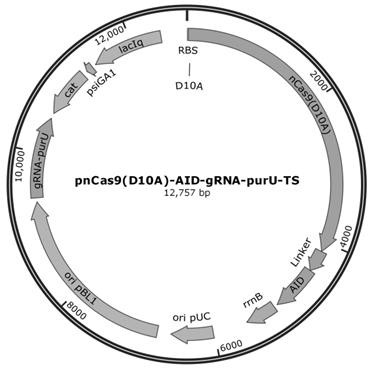
[0038] Example 2 inactivation purU Genetic construction of recombinant Corynebacterium glutamicum
[0039] (1) According to the sequence design of SEQ ID NO.3 for inactivation purU The sgRNA target sequence of the gene (20 bp), synthesize two corresponding sense strand and antisense strand primers (24 bp each) according to the target sequence, and the primer contains a gap sequence (4 bp) introduced at the 5' end and paired with the vector . The two primers are annealed to form double-stranded DNA, and then combined with pnCas9(D10A)-AID-gRNA- ccdB TS Carrier plasmid for Golden Gate connection, transformation E. coli DH5α competent cells were spread onto LB solid plates added with Cm resistance, and cultured overnight in a 37°C incubator. After the monoclonal grows, pick the monoclonal and inoculate it into the LB liquid medium added with Cm resistance, extract the recombinant plasmid from the bacterial solution, and obtain the recombinant plasmid pnCas9(D10A)-AID- g...
Embodiment 3
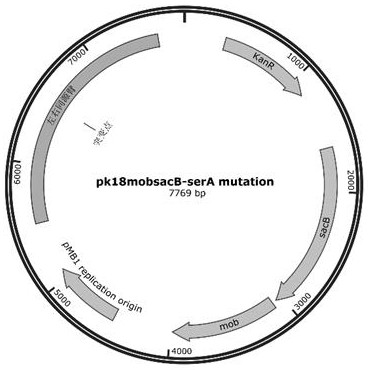
[0041] Example 3 contains serA Construction of recombinant Corynebacterium glutamicum with mutant gene
[0042] 1. Through the suicide plasmid pK18 mobsacB The method of double exchange is constructed, and the steps are as follows:
[0043] (1) with C. glutamicum Genomic DNA of ATCC13032 is used as a template, designed for mutation according to the sequence of SEQ ID NO.4 serA The left and right homology arms of the gene (about 1kb each) and their primer sequences, the mutation site (Q184K) sequence was introduced into the primers, the left and right homology arms were amplified by PCR, the products were purified and recovered, and then combined with enzyme-digested pK18 mobsacB The plasmid fragments are connected by homologous recombination of three fragments, and the connection products are introduced by chemical transformation E. coli DH5α competent cells were spread onto LB solid plates added with Kan resistance, and cultured overnight in a 37°C incubator. Aft...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com