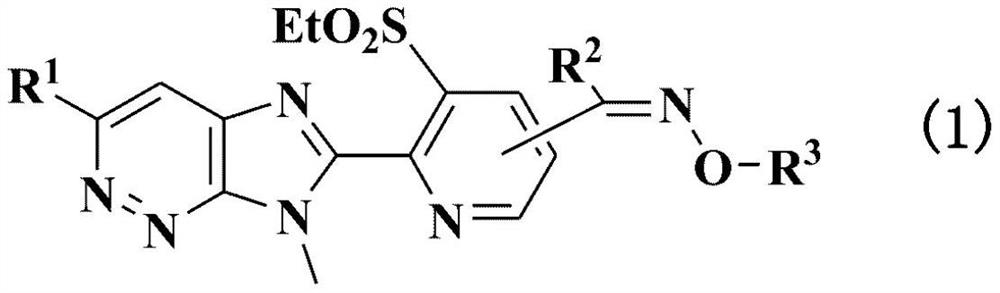
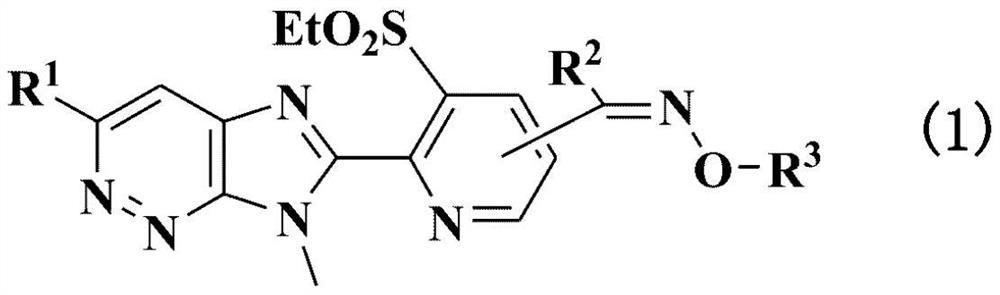
Agricultural and horticultural insecticide or external or internal parasite control agent for animals, containing imidazopyridazine compound or salt thereof as active ingredient, and method for using same
A technology of imidazopyridazine and compounds, which is applied in the field of external or internal parasite control agents, can solve the problems of unspecified compounds and achieve excellent results
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
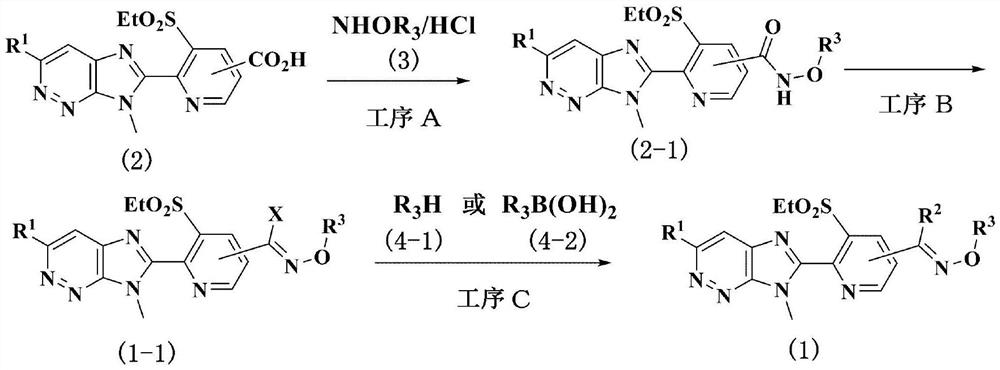
[0050] Preparation method of process A
[0051] The compound represented by the general formula (2-1) can be obtained by combining a carboxylic acid compound represented by the general formula (2) prepared according to the method described in International Publication No. 2017 / 146221 in the presence of a base and an inert solvent. The compound represented by general formula (3) is produced by reacting with a condensing agent.
[0052] Examples of bases that can be used in this reaction include inorganic bases such as sodium hydroxide, potassium hydroxide, sodium carbonate, potassium carbonate, sodium bicarbonate, and potassium bicarbonate; acetates such as potassium acetate; triethylamine , diisopropylethylamine, 1,8-diazabicyclo[5.4.0]undec-7-ene and other tertiary amines; pyridine, 4-dimethylaminopyridine and other nitrogen-containing aromatic compounds, etc. , the amount used is usually within the range of 1-fold mole to 10-fold mole relative to the compound represented by...
reference example 1
[0179] The preparation method of reference example 1.N-methylthioethoxyphthalimide
[0180] [chemical formula 4]
[0181]
[0182] Diethyl azodicarboxylate ( 2.2M solution in toluene, 33.4 mL). After stirring at room temperature for 1 hour, it was concentrated. The residue was subjected to column chromatography to obtain the target N-methylthioethoxyphthalimide (15 g, yield: quantitative).
reference example 2
[0183] The preparation method of reference example 2.methylthioethoxylamine
[0184] [chemical formula 5]
[0185]
[0186] To a mixture of N-ethylthioethoxyphthalimide (15 g), chloroform (60 mL) was added hydrazine monohydrate (3 mL). After stirring at room temperature for 4 hours, the insoluble matter was removed by filtration through celite. After drying the filtrate with magnesium sulfate, it filtered again. The obtained filtrate was made into a 0.3M solution of methylthioethoxyamine and used for the reaction.
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
| control rate | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com