Method for synthesizing heptasaccharide of lentinan core fragment beta-(1-> 6) branched chain beta-(1-> 3) main chain
A technology of core fragments and lentinan, which is applied in chemical instruments and methods, drug combinations, sugar derivatives, etc., can solve the problems of normal cell killing, etc., and achieve the effect of extremely simple route, controllable quality and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
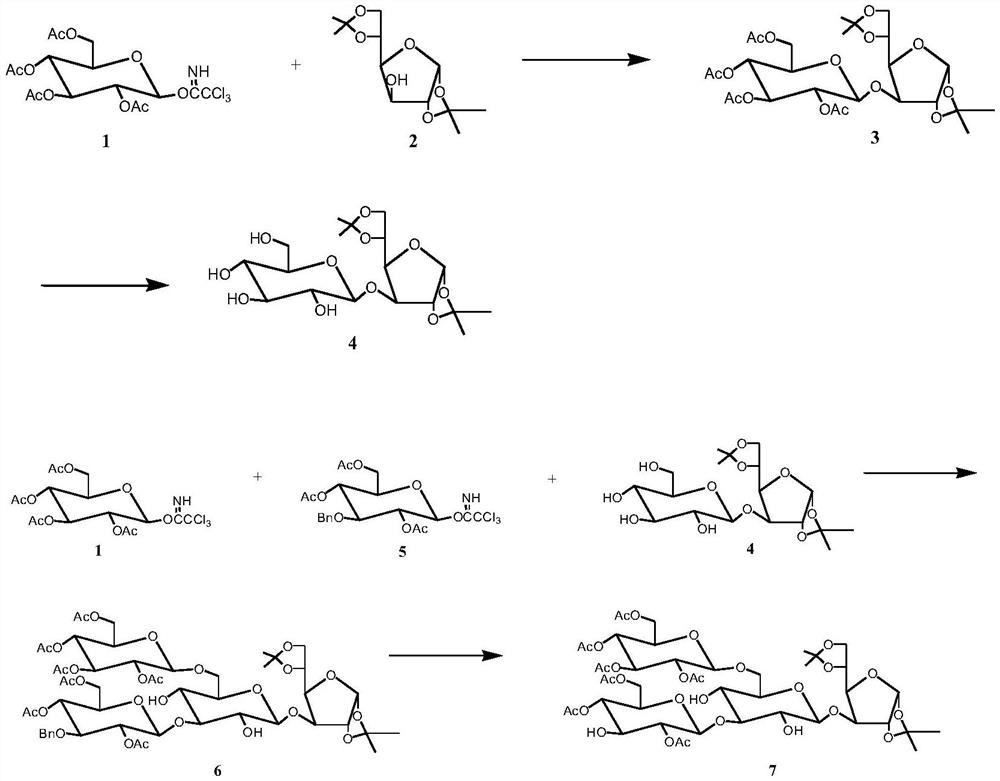
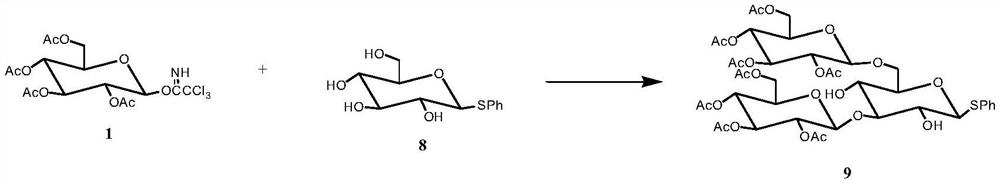
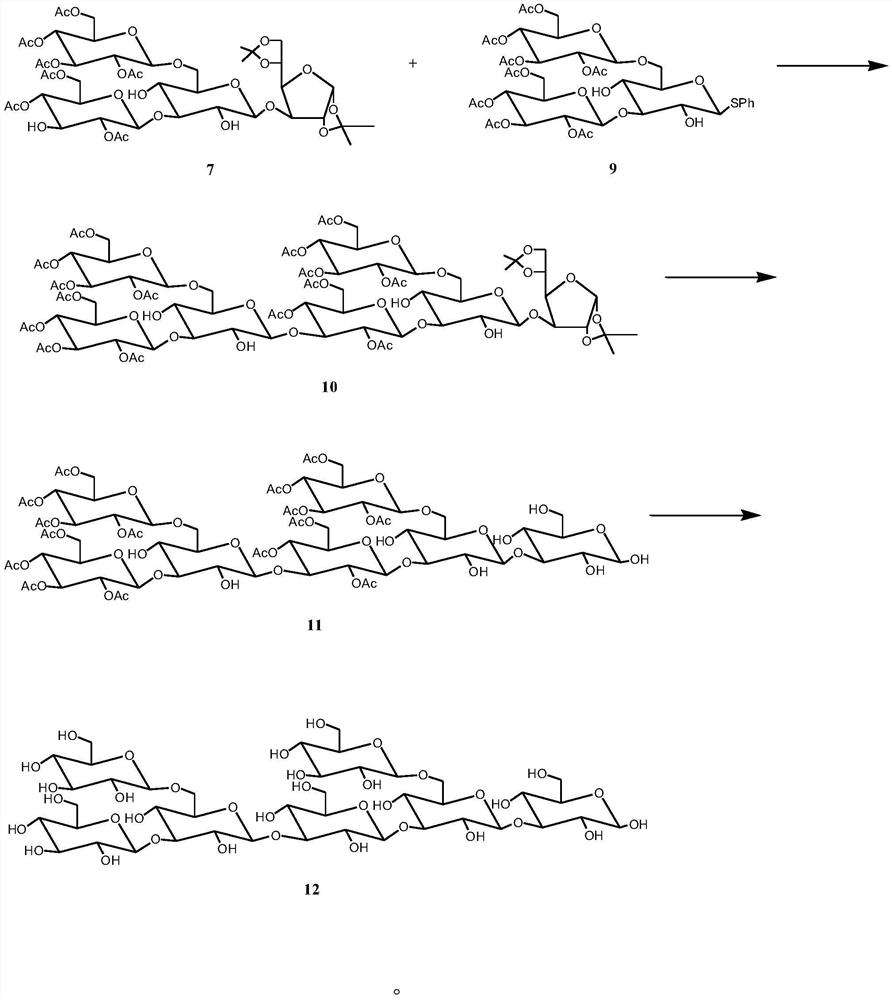
[0039] Preparation of Tetrasaccharide Acceptor 7 and Trisaccharide Donor 9
[0040] (1) 2,3,4,6-Tetra-O-acetylglucose trichloroacetimidate 1 (2.96 g, 6 mmol) was dissolved in 10 mL of dichloromethane to obtain solution A, 1,2- 5,6-Di-O-isopropylideneglucose 2 (1.56 g, 6 mmol) was dissolved in 10 mL of dichloromethane to obtain solution B, A and B were mixed to obtain solution C, to which was added TMSOTf (28 microliters, 0.25 mmol), stirring at 25°C, and reacting for 2-4 hours, TLC analysis showed that the reaction was complete. The solvent was distilled off under reduced pressure, and disaccharide 3 was obtained through coupling. Dissolve disaccharide 3 (3.54g, 5.4 mmol) in 20 ml of alcoholamine-dichloromethane, stir at 25°C, and react for 8-10 hours. TLC analysis shows that the reaction is complete, and the solvent is distilled off under reduced pressure to obtain Disaccharide 4, the yield was 94.1%.
[0041] (2) 2,3,4,6-Tetra-O-acetylglucose trichloroacetimidate 1 (2.96 ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com