Alkyl aromatic hydrocarbon non-hydroisomerization catalyst as well as preparation method and application thereof
A technology for alkyl aromatic hydrocarbons and catalysts, applied in the field of non-hydrogen isomerization catalysts for alkyl aromatic hydrocarbons and its preparation, capable of solving problems such as reduced reaction selectivity, achieving high selectivity, energy saving, and high isomerization activity Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0069] (1) Preparation of catalyst carrier
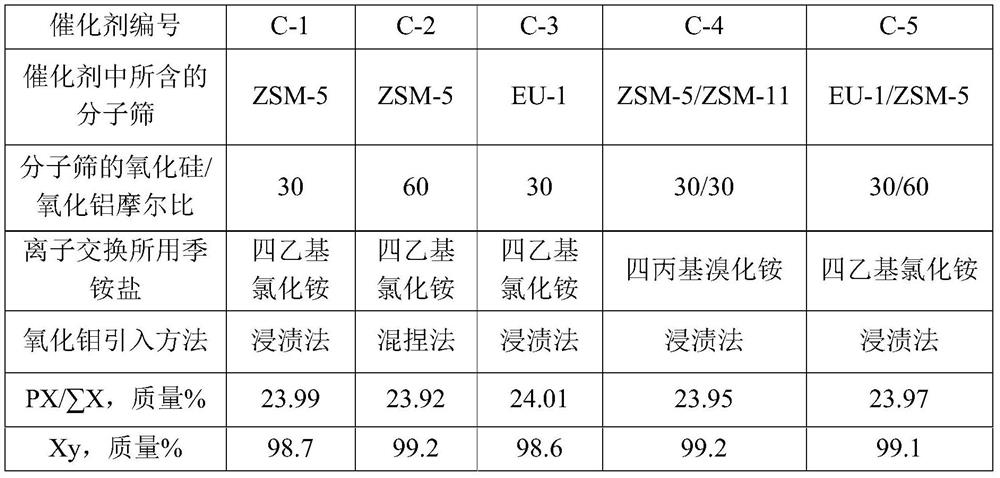
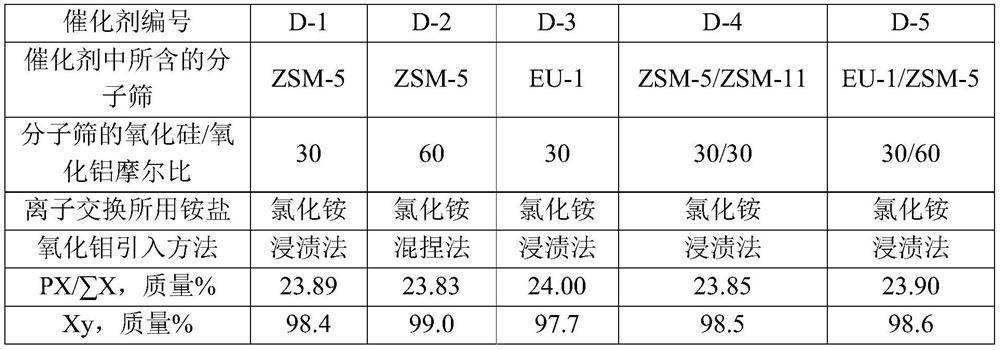
[0070] Take SiO 2 / Al 2 o 3 The Na-type ZSM-5 molecular sieve with a molar ratio of 30, its Na 2 The content of O is 0.7 mol%, and it is mixed with alumina powder at a dry basis mass ratio of 70:27. Add 40% of the total mass of the powder with a concentration of 2% nitric acid aqueous solution, knead evenly, extrude, dry at 120°C for 2 hours, and roast at 580°C for 3 hours in air to obtain a catalyst carrier.
[0071] (2) Ion exchange the catalyst carrier
[0072] Get 10 grams of the catalyst carrier that (1) step makes, use the tetraethylammonium chloride aqueous solution of 3 mass % with 20 gram concentrations to carry out ion exchange at 90 ℃ for 2 hours, the solid after ion exchange is washed with deionized water until There is no chlorine ion in the washing liquid, and the catalyst carrier after ion exchange is obtained.
[0073] (3) Preparation of catalyst
[0074]The catalyst support after the ion exchange that (2) ste...
example 2
[0076] (1) Preparation of catalyst precursor
[0077] Take SiO 2 / Al 2 o 3 The Na-type ZSM-5 molecular sieve with a molar ratio of 60, its Na 2 The content of O is 0.7 mol%, and it is mixed with alumina and molybdenum oxide at a dry basis mass ratio of 70:27:3. Add 40% of the total mass of the powder with a concentration of 2% nitric acid aqueous solution, knead evenly, extrude, dry at 120° C. for 2 hours, and roast at 580° C. in air for 3 hours to obtain a catalyst precursor.
[0078] (2) Preparation of catalyst
[0079] Take 10 grams of the catalyst precursor prepared in step (1), and perform ion exchange at 90° C. for 2 hours with 20 grams of tetraethylammonium chloride aqueous solution with a concentration of 3% by mass. Wash the ion-exchanged solid with deionized water until there is no chloride ion in the washing liquid, then dry at 60°C for 6 hours, and roast at 500°C in air for 4 hours to obtain catalyst C-2, wherein the content of hydrogen ZSM-5 molecular sieve ...
example 3
[0081] Catalyst C-3 is prepared by the method for example 1, and difference is that the Na type ZSM-5 molecular sieve used in (1) step is changed into SiO 2 / Al 2 o 3 The Na-type EU-1 molecular sieve with a molar ratio of 30, its Na 2 The O content was 0.2 mol%, and the prepared catalyst C-3 contained 70% by mass of hydrogen-type EU-1 molecular sieve, 3% by mass of molybdenum oxide, and 27% by mass of alumina.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com