Synthesis method of methyl thiomethylene ester compound
A technology of methyl thiomethylene ester and synthesis method, applied in the directions of organic chemistry, sulfide preparation, etc., can solve problems such as unfavorable industrial production, hydrogen generation, expensive electrodes, etc., and achieve cost saving, simple production, and reaction yield. high rate effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
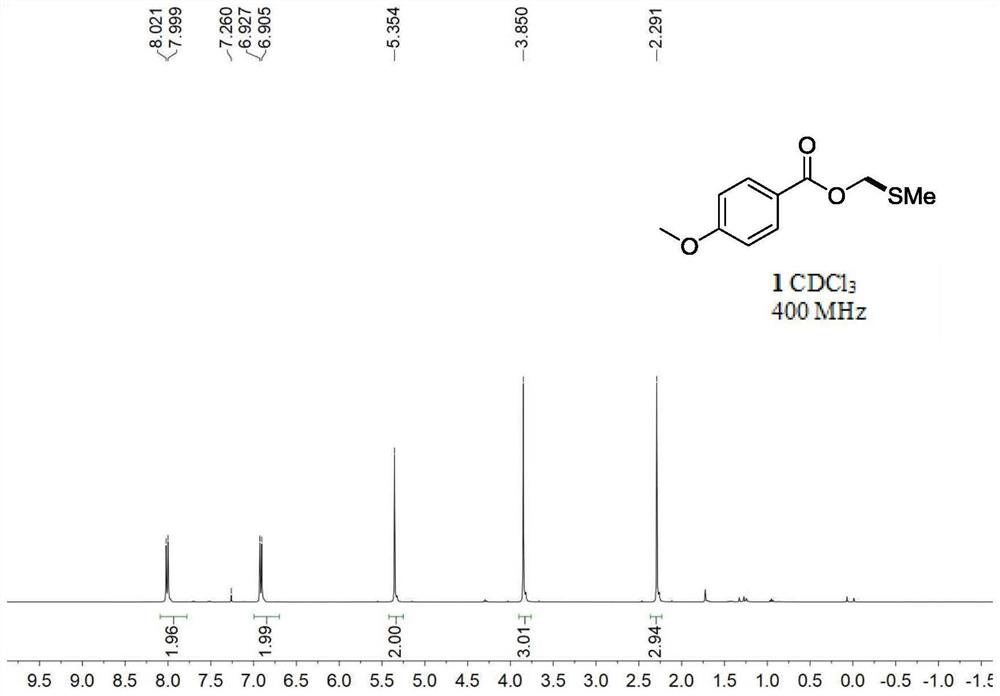
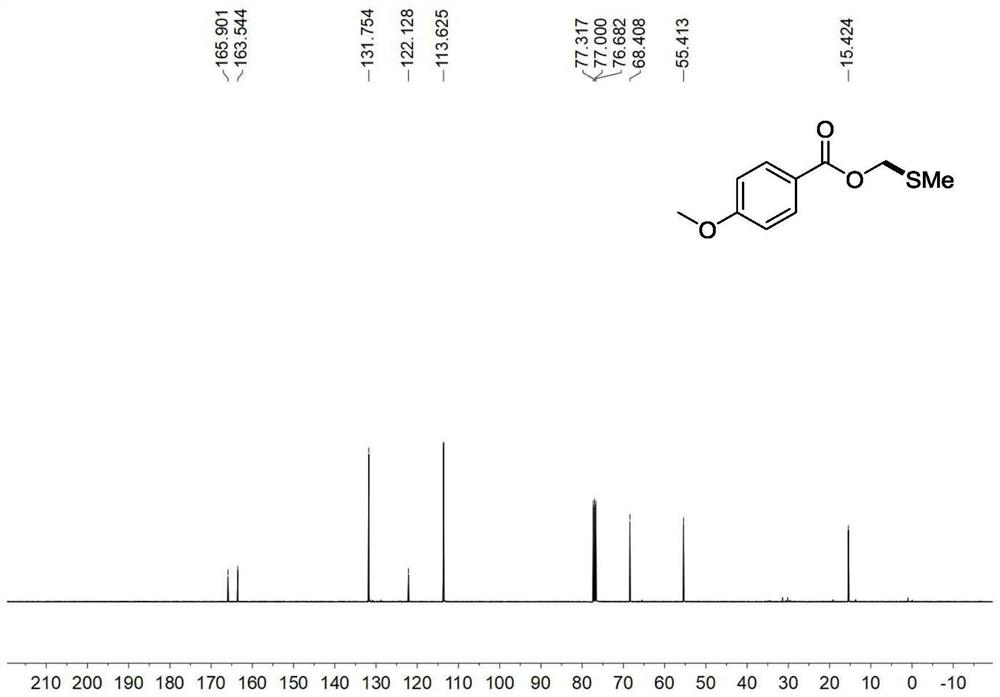
[0047] Embodiment 1: the synthesis of p-methoxybenzoic acid methylsulfomethylene ester
[0048] Add p-methoxybenzoic acid (45.6mg, 1.0eq), dimethylsulfonium bromide (100mg, 1.5eq), triethylamine (91.1mg, 125uL, 3.0eq ) and 3 mL of acetonitrile. In a nitrogen atmosphere, the temperature was gradually raised to room temperature at 0° C. and reacted for 18 h. After the reaction was completed, distilled water was added to quench the reaction, extracted 3 times with ethyl acetate, 10 mL each time, the combined organic phase was concentrated by rotary evaporation, and then column chromatography was carried out to obtain methylsulfomethylene p-methoxybenzoate The ester was 57.5 mg, and the yield was 90%.
[0049] Product Methylsulfomethylene p-methoxybenzoate: 1 H NMR (400MHz, CDCl 3 )δ8.03(d, J=8.8Hz, 2H), 6.94(d, J=8.8Hz, 2H), 5.35(s, 2H), 3.85(s, 3H), 2.29(s, 3H). 13 C NMR (101MHz, CDCl 3 )δ165.9, 163.5, 131.75, 122.1, 113.6, 68.4, 55.4, 15.4.
Embodiment 2
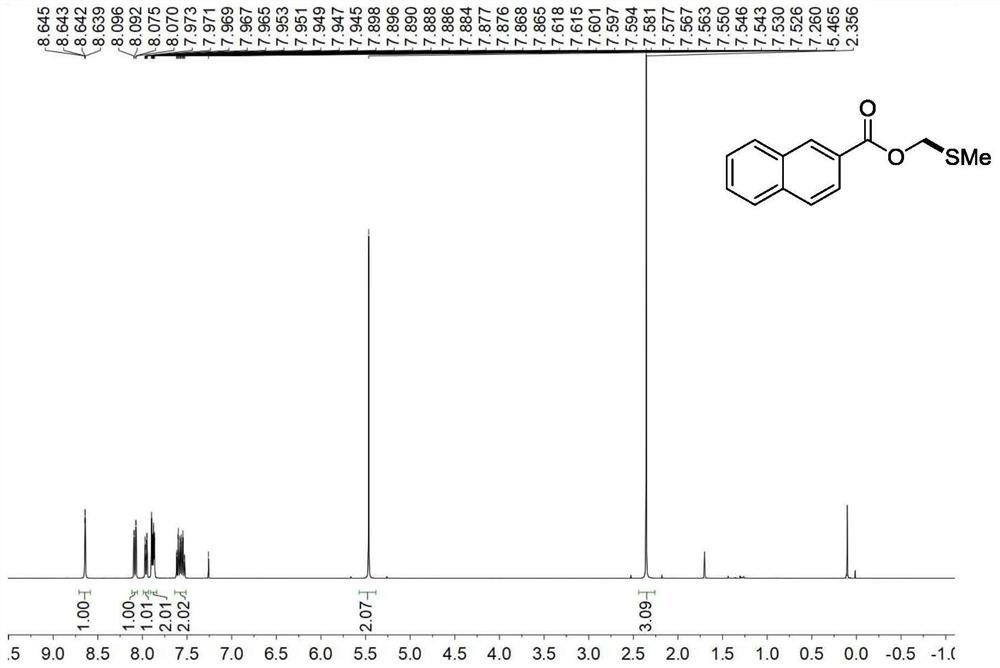
[0050] Embodiment 2: the synthesis of 2-naphthoic acid methylsulfomethylene ester
[0051] Add 2-naphthoic acid (51.7mg, 1.0eq), dimethylsulfonium bromide (100mg, 1.5eq), triethylamine (91.1mg, 125uL, 3.0eq) and 3 mL of acetonitrile. In a nitrogen atmosphere, the temperature was gradually raised to room temperature at 0° C. and reacted for 18 h. After the reaction was completed, distilled water was added to quench the reaction, extracted 3 times with ethyl acetate, 10 mL each time, the combined organic phase was concentrated by rotary evaporation, and then column chromatography was carried out to obtain 2-naphthoic acid methylsulfomethylene ester 60.0 mg, the yield was 86%.
[0052] Product 2-Naphthoic acid methylsulfomethylene ester: 1 H NMR (400MHz, CDCl 3 )δ8.64(s,1H),8.12–8.03(m,1H),7.99–7.94(m,1H),7.91–7.85(m,2H),7.63–7.51(m,2H),5.46(s, 2H), 2.36(s, 3H). 13 C NMR (101MHz, CDCl 3 )δ166.3, 135.6 132.3, 131.3 129.3, 128.4, 128.2, 127.7, 126.9, 126.7, 125.1, 68.9 15.5....
Embodiment 3
[0053] Embodiment 3: the synthesis of piperic acid methylsulfomethylene ester
[0054] Add piperic acid (50.0 mg, 1.0 eq), dimethylsulfonium bromide (100 mg, 1.5 eq), triethylamine (91.1 mg, 125 uL, 3.0 eq) and 3 mL of acetonitrile into a Schlenk reaction tube equipped with a magnetic stir bar . In a nitrogen atmosphere, the temperature was gradually raised to room temperature at 0° C. and reacted for 18 h. After the reaction was completed, distilled water was added to quench the reaction, and ethyl acetate was used to extract 3 times, 10 mL each time, the combined organic phase was concentrated by rotary evaporation, and then column chromatography was carried out to obtain 57.3 mg of methylsulfomethylene piperonate. The yield was 88%.
[0055] Product Methylsulfomethylene piperonate: 1 H NMR (400MHz, CDCl 3 )δ7.71–7.56(m,1H),7.45(d,J=1.8Hz,1H),6.82(d,J=8.2Hz,1H),6.02(s,2H),5.33(s,2H), 2.27(s,3H). 13 C NMR (101MHz, CDCl 3 )δ165.4, 151.8, 147.6, 125.5, 123.6, 109.4, 107....
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com