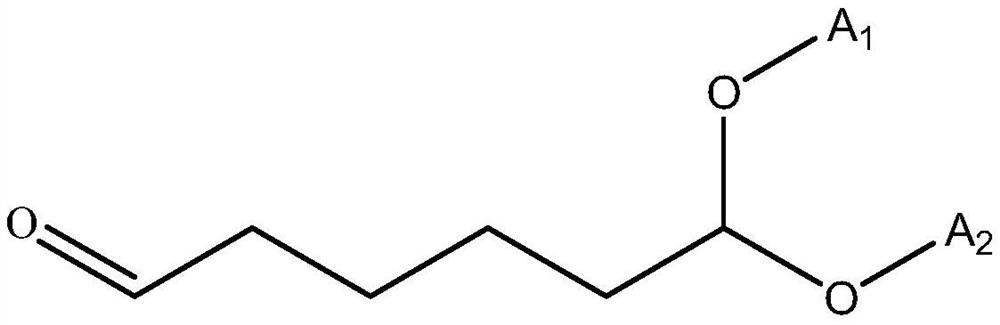
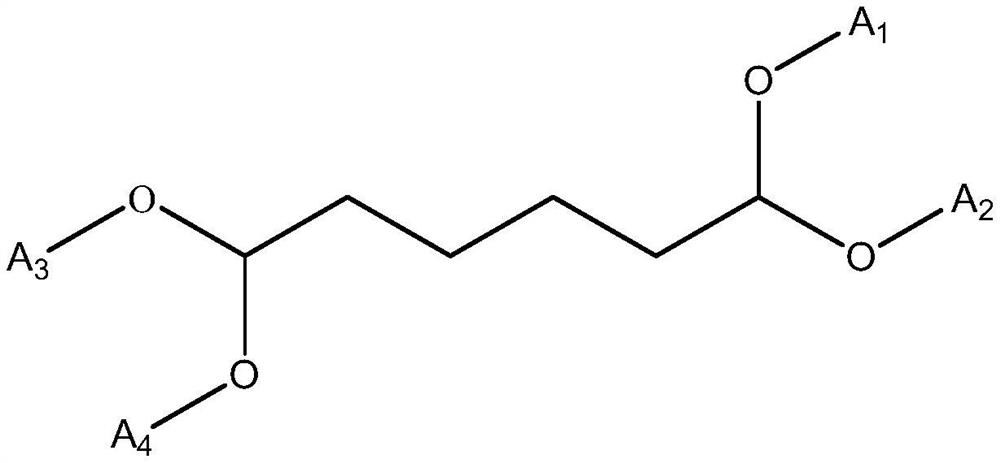
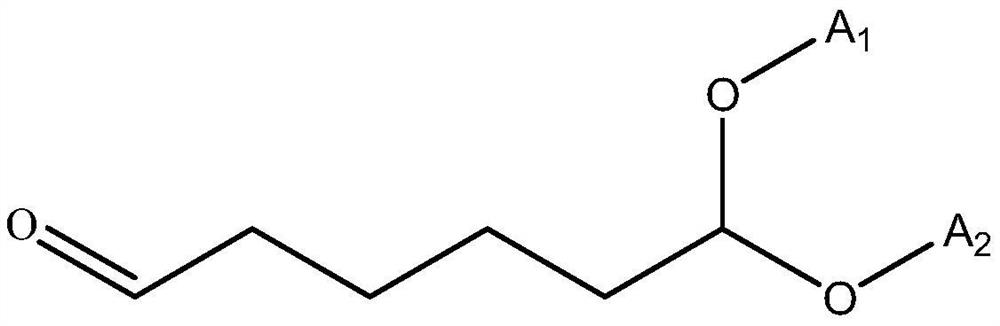
Cycloheximine synthesis method, adipic dialdehyde selective ammoniation hydrogenation catalyst and preparation method of adipic dialdehyde selective ammoniation hydrogenation catalyst
A technology of cyclohexyl imine and synthesis method, which is applied in the direction of catalyst activation/preparation, metal/metal oxide/metal hydroxide catalyst, physical/chemical process catalyst, etc., and can solve the problem of high cost of cyclohexyl imine and the price of caprolactam Expensive and other issues, to achieve the effect of good catalytic effect and easy preparation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0039] A preparation method for adipaldehyde selective ammoniation hydrogenation catalyst, comprising the following steps:
[0040] a) using an impregnation method to impregnate the precursor salt of Ru and the modified metal component on the carrier;
[0041] b) drying the impregnated mixture in step a) at 100-130 degrees;
[0042] c) using hydrogen to reduce the solid obtained in step b) at 300-500 degrees;
[0043] The precursor salt of Ru and the modified metal component is a nitrate or chloride salt of Ru and the modified metal component; the carrier is selected from alumina, zirconia, magnesium oxide, titanium dioxide, niobium oxide and oxide One or a mixture of vanadium.
[0044] In the preparation method of the catalyst provided by the present invention, wherein the weight ratio of the Ru precursor salt, the modified metal precursor salt and the carrier is such that in the prepared catalyst, based on the total weight of the catalyst, in terms of metal, The content o...
Embodiment 1
[0051] Catalyst A preparation method is as follows:
[0052] a) RuCl containing 0.1g of Ru 3 and AgNO containing Ag0.02g 3Dissolve in 20ml aqueous solution, impregnate equal volume into 5g carrier Al 2 o 3 superior;
[0053] b) drying the impregnated mixture in step a) at 110°C;
[0054] c) The solid obtained in the hydrogen reduction step b) at 420 degrees is the catalyst A.
[0055] The composition of the catalyst is: the content of Ru is 2wt%, the content of Ag is 0.4wt%, and the rest is Al 2 o 3 .
Embodiment 2
[0057] Catalyst B preparation method is as follows:
[0058] a) RuCl containing 0.1g of Ru 3 and Cu(NO 3 ) 2 Dissolve in 20ml aqueous solution, impregnate equal volume into 10g carrier Al 2 o 3 superior;
[0059] b) drying the impregnated mixture in step a) at 120 degrees;
[0060] c) The solid obtained in the hydrogen reduction step b) at 400 degrees is the catalyst B.
[0061] The composition of the catalyst is: the content of Ru is 10wt%, the content of Cu is 2wt%, and the rest is Al 2 o 3 .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com