Artemisinin/artemisinin derivative solid dispersion as well as preparation method and application thereof
A technology of artemisinin derivatives and solid dispersion, which is applied in the field of medicine to achieve high solubility and improve solubility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
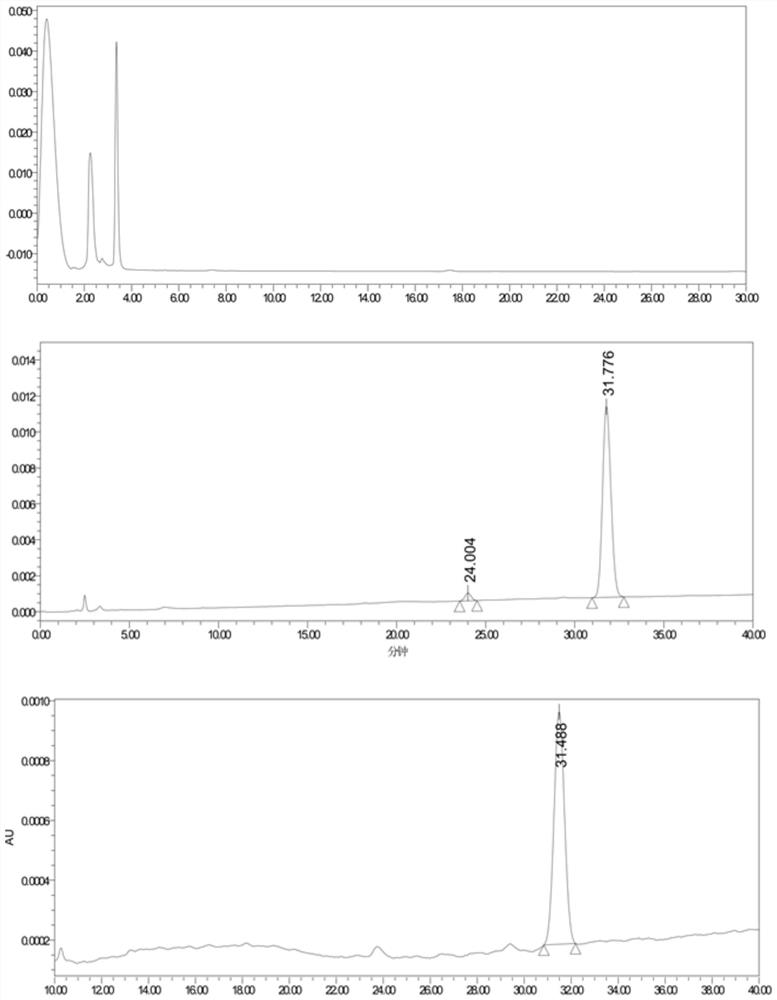
[0033] 3, measure the preparation method of solubility need testing solution
[0034] Take the raw material drug and the solid dispersion preparation, put them in a 5mL centrifuge tube, add about 3mL of water, shake on a shaker at 37°C at 150r / min for more than 12 hours, and filter through a 0.22μm filter membrane.
[0035] 4. The stability of raw materials at different temperatures and times
[0036] The raw materials of artemisinin and dihydroartemisinin were taken respectively, and samples were taken at different temperatures and at different time points to determine the stability of artemisinin and dihydroartemisinin. Results The content of artemisinin was basically stable when heated at 80°C for 6 hours, and the content of dihydroartemisinin was unstable at 80°C. Although the content measurement results showed no obvious change, it had turned pale yellow after heating for 6 hours; 60°C and 70°C When heated at ℃ for 8 hours, artemisinin and dihydroartemisinin were relativ...
Embodiment 1
[0039] Embodiment 1: the preparation of solid dispersion preparation and the mensuration of solubility
[0040] 1. Process comparison of solid dispersion prepared by solvent method
[0041] 1.1, the choice of solvent
[0042] The carrier materials selected in this study (F68, PVP10, PEG2000, PEG4000, PEG6000, PVP K30) were all easily soluble in absolute ethanol. Based on solvent toxicity and cost considerations, absolute ethanol was selected as the solvent, and the drying method was 12 hours under reduced pressure at 60°C.
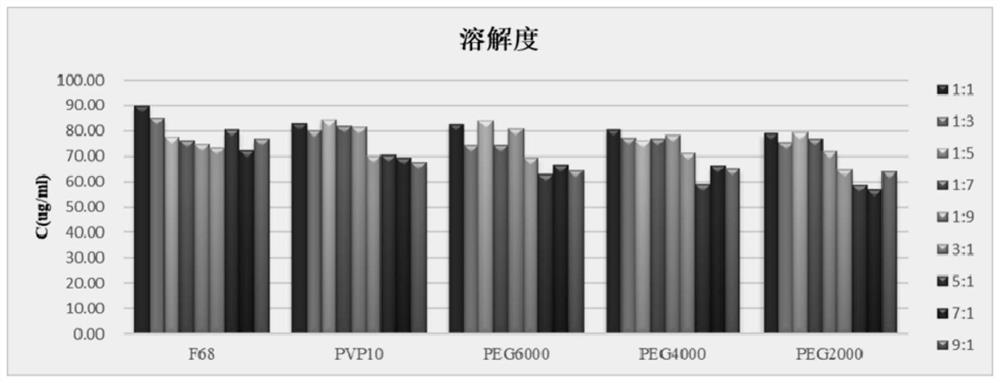
[0043] 1.2. Comparison of excipient types and excipient-drug ratio
[0044] The saturated solubility of artemisinin in water is used as the evaluation index, and the types of excipients and the ratio of excipients to drugs are compared. The results are shown in image 3 , the results of different excipients and ratios showed that the drug-adjuvant ratio was 1:1, and the solubility of the solid dispersion was the best, and the solubility of the solid dis...
Embodiment 2
[0057] Example 2: IR Characterization of Artemisinin and Dihydroartemisinin Solid Dispersions
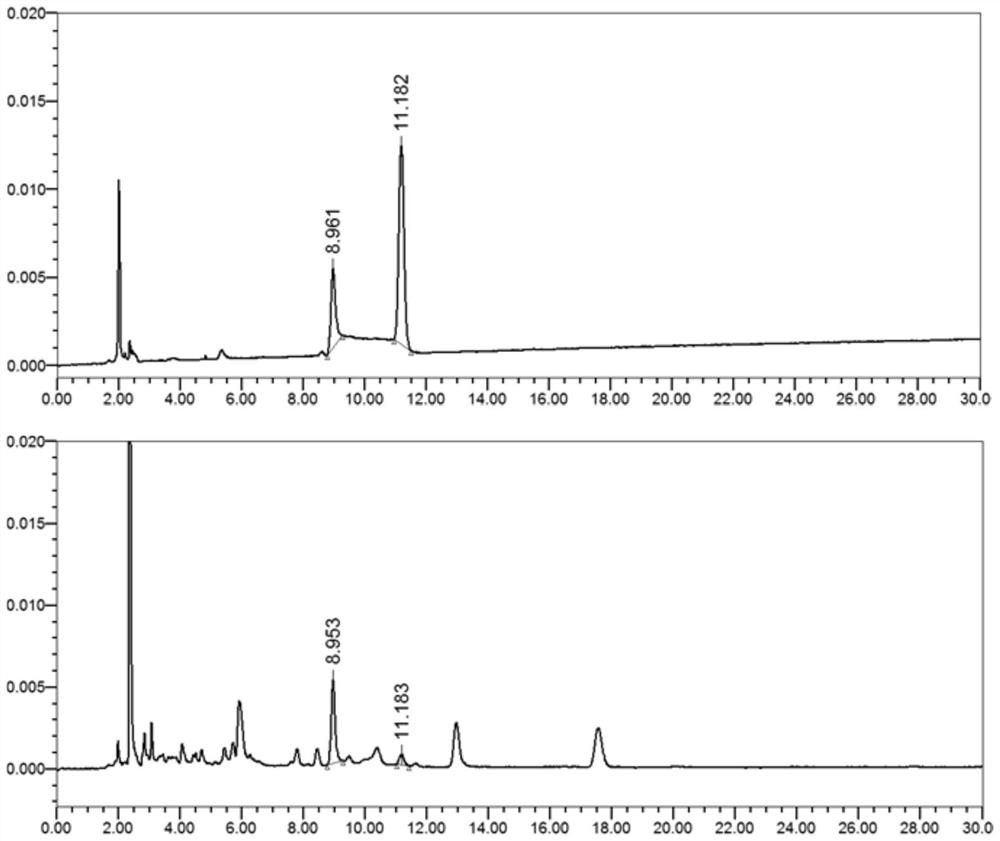
[0058] Take artemisinin API, dihydroartemisinin API, poloxamer 188, artemisinin + poloxamer 188 physical mixture, dihydroartemisinin + poloxamer 188 physical mixture, artemisinin The solid dispersion sample and the dihydroartemisinin solid dispersion sample were subjected to IR measurement, and the results are shown in Figure 6-12 .
[0059] Depend on Figure 6-12 It can be seen that the physical mixture of artemisinin and dihydroartemisinin raw materials and excipients is a physical superposition of raw materials and excipients, and the IR spectrum of the solid dispersion preparation shows no new peaks, indicating that the raw materials and excipients have not interacted.
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap