Application of geniposide in preparation of medicine for treating psoriasis
A technology for psoriasis and geniposide, which is applied in the application field of medicine, can solve the problems of increased incidence and achieve the effect of improving skin lesions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Pharmacodynamic experiment of imiquimod-induced psoriasis model
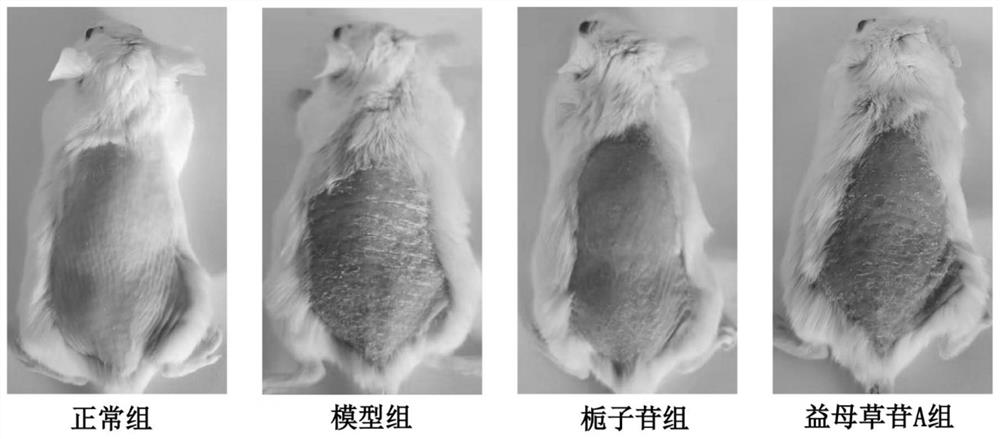
[0031] Animals and grouping: 12 SPF grade BALB / c mice, male, 8 weeks old, purchased from Guangdong Medical Experimental Animal Center, production license number SCXK (Guangdong) 2013-0002. The experimental animals were kept in the barrier environment animal room of Guangdong Provincial Hospital of Traditional Chinese Medicine. This experiment was approved by the Ethics Committee of Guangdong Provincial Hospital of Traditional Chinese Medicine (NO.2021090). Twelve BALB / c mice were randomly divided into four groups according to body weight: normal group, model group, geniposide group, and leonurin A group, with 3 mice in each group.
[0032] Drugs and reagents: In this experiment, the iridoid components geniposide and motherrubioside A were selected. The batch numbers are S14J10Y79596 and J11GB154353 respectively. They were purchased from Shanghai Yuanye Biotechnology Co., Ltd. and prepared by adding pure ...
Embodiment 2
[0043] Anti-psoriasis efficacy experiment of geniposide
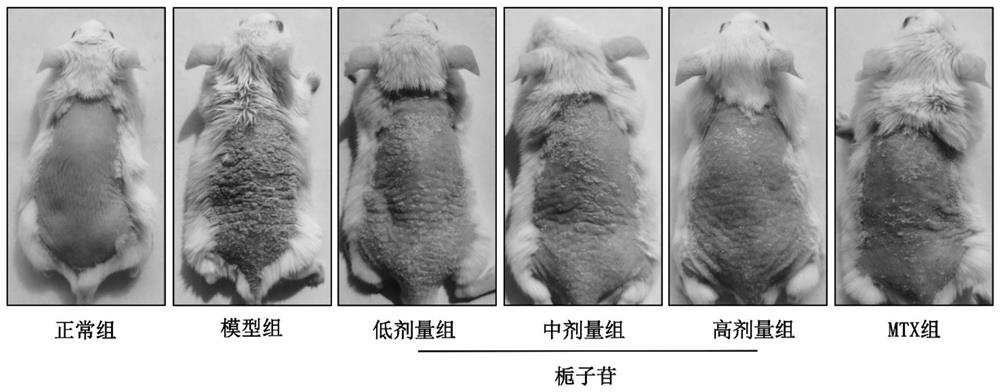
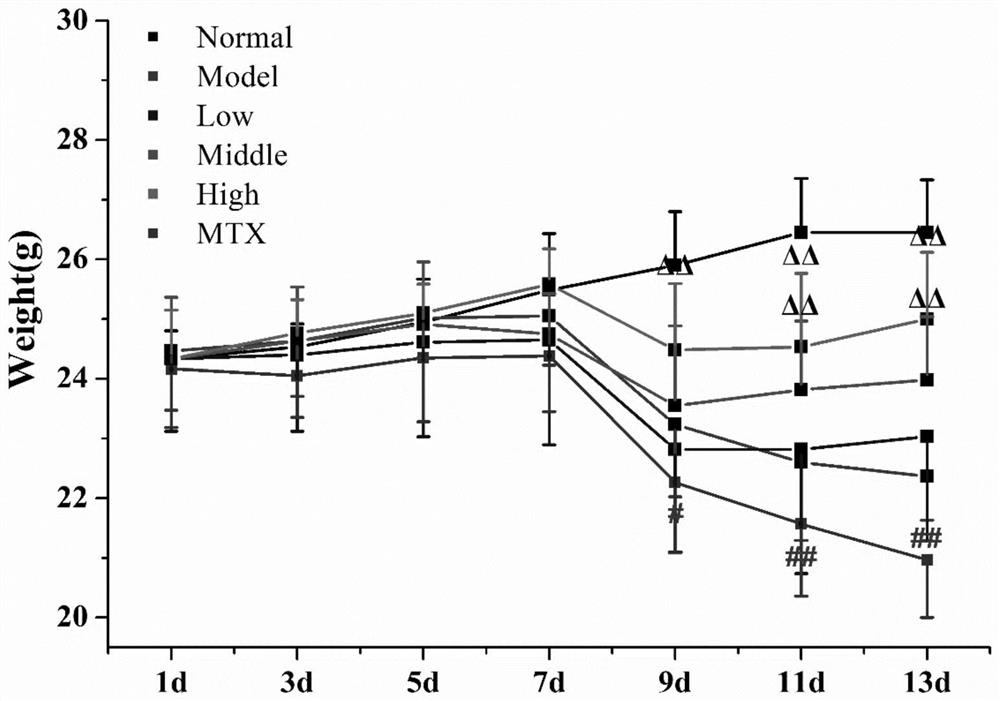
[0044] Animals and grouping: 48 SPF grade BALB / c mice, male, 8 weeks old, were purchased from Guangdong Medical Experimental Animal Center, production license number SCXK (Guangdong) 2013-0002. The experimental animals were kept in the barrier environment animal room of Guangdong Provincial Hospital of Traditional Chinese Medicine, the room temperature was 20-25°C, the relative humidity was 40%-70%, and the light and dark alternated between day and night for 12h / 12h. This experiment was approved by the Ethics Committee of Guangdong Provincial Hospital of Traditional Chinese Medicine (NO.2021090). Forty-eight BALB / c mice were randomly divided into normal group, model group, geniposide low-dose, medium-dose and high-dose groups, and methotrexate group according to body weight, a total of 6 groups with 8 mice in each group.
[0045] Drugs and reagents: methotrexate tablets, 2.50 mg per tablet, Shanghai Xinyi Pharmaceutica...
Embodiment 3
[0073] A tablet for the treatment of psoriasis
[0074] prescription:
[0075]
[0076] Preparation method: Weigh the geniposide raw material drug, lactose and sodium hydroxymethyl starch (75% of the prescription quantity) according to the prescription quantity, mix them, pass through a 120-mesh sieve for 3 times, and mix well. Add an appropriate amount of binder to make a soft material. The soft material is passed through a 18-mesh sieve, and the wet granules are dried in an oven at 50°C for 1 hour. Prescription amount), mix well, compress into tablets, and get ready.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com