Preparation method of iopromide intermediate
A technology for iopromide and intermediates, applied in the field of preparation of iopromide intermediates, can solve the problems of large amount of organic solvent, low yield, long route, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
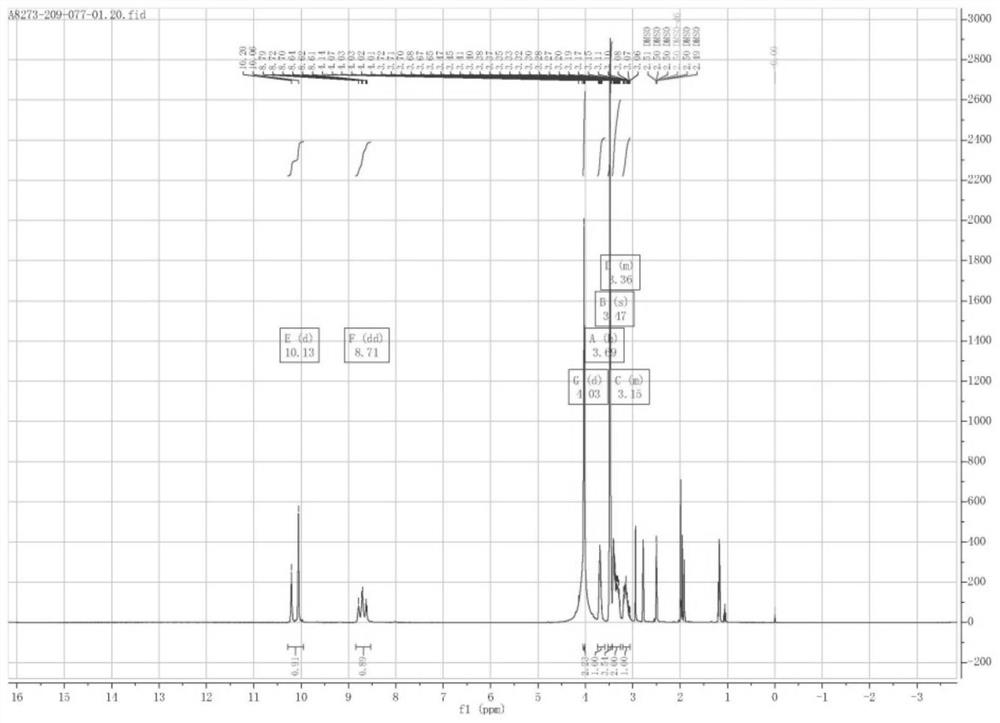
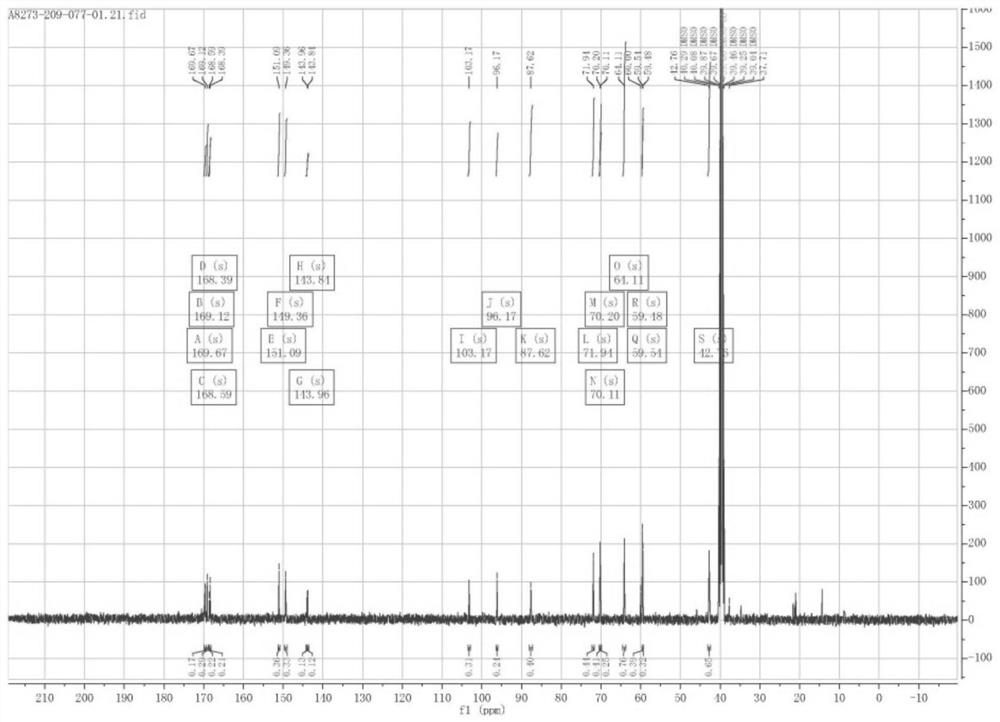
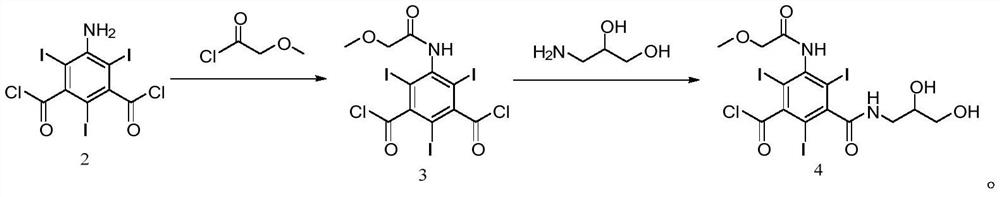
[0069] 100g (0.17mol) of the starting material was dissolved by adding 100ml DMA, and 27.3g (0.25mol) of methoxyacetyl chloride was added dropwise at 20°C for about 1 hour, and kept at 20-30°C for 5-8h. Cool down to 5°C, add 1g (0.06mol) of water, slowly add 58.7g (0.42mol) of potassium carbonate, continue to add 400ml of dichloromethane, slowly add 17.8g (0.2mol) of 3-amino-1,2-propanediol dropwise, drop After the addition was completed and the reaction was completed for about 8 hours, the reaction solution was quickly added to 400ml of cold water, crystallized at 0-5°C for 2 hours, filtered, washed with a small amount of water, and dried to obtain 118.1g of compound 4 with a yield of 97.4% and a main peak of 99.4%.
[0070] The obtained compound 4 is characterized by structure:
[0071]
[0072] 1 H NMR (400MHz, DMSO-d 6 )δ10.13 (d, J=58.7Hz, 1H, -NHAr), 8.71 (dd, J=35.9, 29.2Hz, 1H, -NHCO), 4.03 (d, J=2.8Hz, 2H, H-13) ,3.69(h,J=5.4Hz,1H,H-9),3.47(s,4H,H-14andH-8a),3.4...
Embodiment 2
[0075] 100g (0.17mol) of the starting material was dissolved by adding 100ml of DMF, and 27.3g (0.25mol) of methoxyacetyl chloride was added dropwise at 20-25°C for about 1 hour, and kept at 20-30°C for 5-8h. Cool down to 0°C, add 1g (0.06mol) of water, slowly add 58.7g (0.42mol) of potassium carbonate, continue to add 400ml of ethyl acetate, slowly add 17.8g (0.2mol) of 3-amino-1,2-propanediol, dropwise After the addition was completed and the reaction was completed for about 8 hours, the reaction solution was quickly added to 400ml of cold water, crystallized at -5 to 0°C for 2 hours, filtered, washed with a small amount of water, and dried to obtain 115.8g of compound 4, with a yield of 95.5% and a main peak of 99.3%.
Embodiment 3
[0077] 100g (0.17mol) of the starting material was dissolved by adding 100ml of DMA, and 27.3g (0.25mol) of methoxyacetyl chloride was added dropwise at 20°C for about 1 hour, and the reaction was carried out at 25°C for 5-8h. Cool down to 0°C, add 1g (0.06mol) of water, slowly add 58.7g (0.42mol) of potassium carbonate, continue to add 600ml of dichloromethane, slowly add 17.8g (0.2mol) of 3-amino-1,2-propanediol, dropwise After the addition was completed and the reaction was completed for about 8 hours, the reaction solution was quickly added to 600ml of cold water, crystallized at 0-5°C for 2 hours, filtered, washed with a small amount of water, and dried to obtain 116.1g of compound 4 with a yield of 95.7% and a main peak of 99.1%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com