Method for preparing cyclic ether through dehydration cyclization of diol
A technology of dehydration cyclization and cyclic ether, which is applied in the field of catalysis, can solve problems such as research and technology that have not been reported, and achieve the effects of no metal participation, high product yield, and strong industrial application value
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
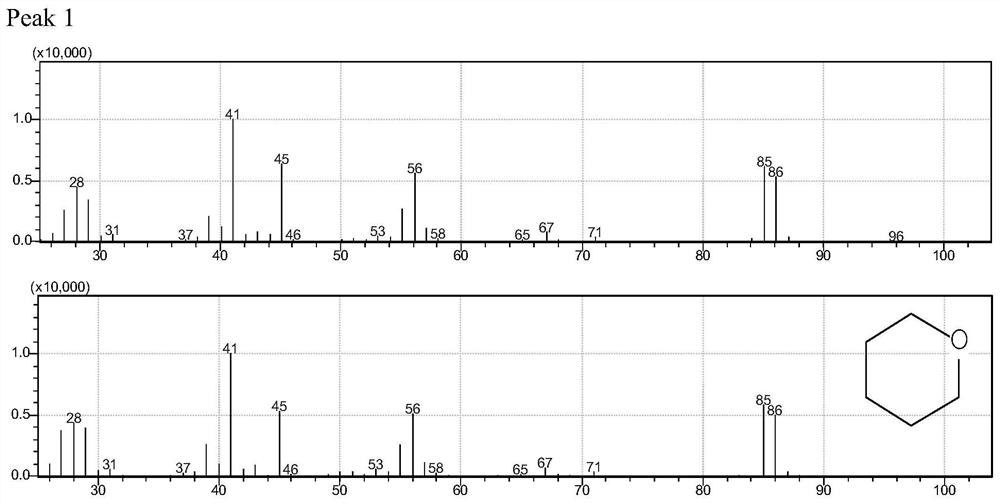
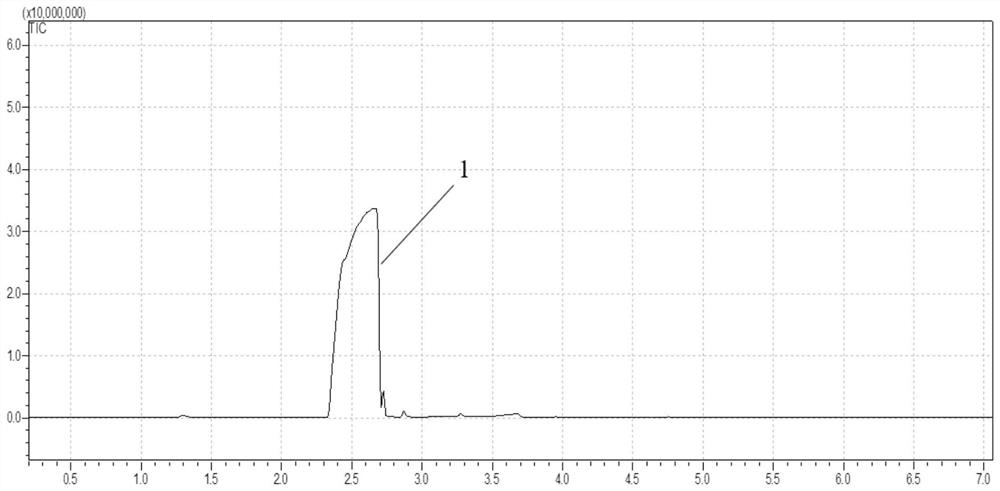
[0057] Embodiment 1, ionic liquid [SO 3 H-BMIm][OTf] Catalyzed Dehydration of 1,4-Butanediol to Tetrahydrofuran
[0058] Put 0.05 moles of ionic liquid and 0.2 moles of 1,4-butanediol in a 20 ml temperature-resistant glass reaction tube, seal it; move it to an oil bath at 120°C, stir and heat for 12 hours for dehydration and cyclization reaction; immerse the reaction tube in The reaction was terminated in ice water, and then it was placed at room temperature for a period of time; then, the phase composition of tetrahydrofuran was analyzed by gas chromatography, and the phase composition of ionic liquid was analyzed by nuclear magnetic resonance. According to the analysis results, the conversion rate of 1,4-butanediol is 100%, the selectivity of THF is 100%, and the yield is >99%.
Embodiment 2
[0059] Example 2, ionic liquid [HO-BMIm][OTf] catalyzed dehydration of 1,4-butanediol to prepare tetrahydrofuran
[0060] Put 0.05 moles of ionic liquid and 0.2 moles of 1,4-butanediol in a 20 ml temperature-resistant glass reaction tube, seal it; move it to an oil bath at 120°C, stir and heat for 12 hours for dehydration and cyclization reaction; immerse the reaction tube in The reaction was terminated in ice water, and then it was placed at room temperature for a period of time; then, the phase composition of tetrahydrofuran was analyzed by gas chromatography, and the phase composition of ionic liquid was analyzed by nuclear magnetic resonance. According to the analysis results, the conversion rate of 1,4-butanediol is 100%, the selectivity of THF is 100%, and the yield is >99%.
Embodiment 3
[0061] Embodiment 3, ionic liquid [SO 3 H-PrMIm][OTf] Catalyzed Dehydration of 1,4-Butanediol to Tetrahydrofuran
[0062] Put 0.05 moles of ionic liquid and 0.2 moles of 1,4-butanediol in a 20 ml temperature-resistant glass reaction tube, seal it; move it to an oil bath at 120°C, stir and heat for 12 hours for dehydration and cyclization reaction; immerse the reaction tube in The reaction was terminated in ice water, and then it was placed at room temperature for a period of time; then, the phase composition of tetrahydrofuran was analyzed by gas chromatography, and the phase composition of ionic liquid was analyzed by nuclear magnetic resonance. According to the analysis results, the conversion rate of 1,4-butanediol is 100%, the selectivity of THF is 100%, and the yield is >99%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com