A β-globin recombinant lentiviral vector and its application
A technology of recombinant lentivirus and globin, which is applied in the field of molecular biology, can solve the problems of high production cost of vectors and low titer of lentivirus packaging, and achieve the effects of reducing potential carcinogenic risk, low production cost and high packaging titer
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
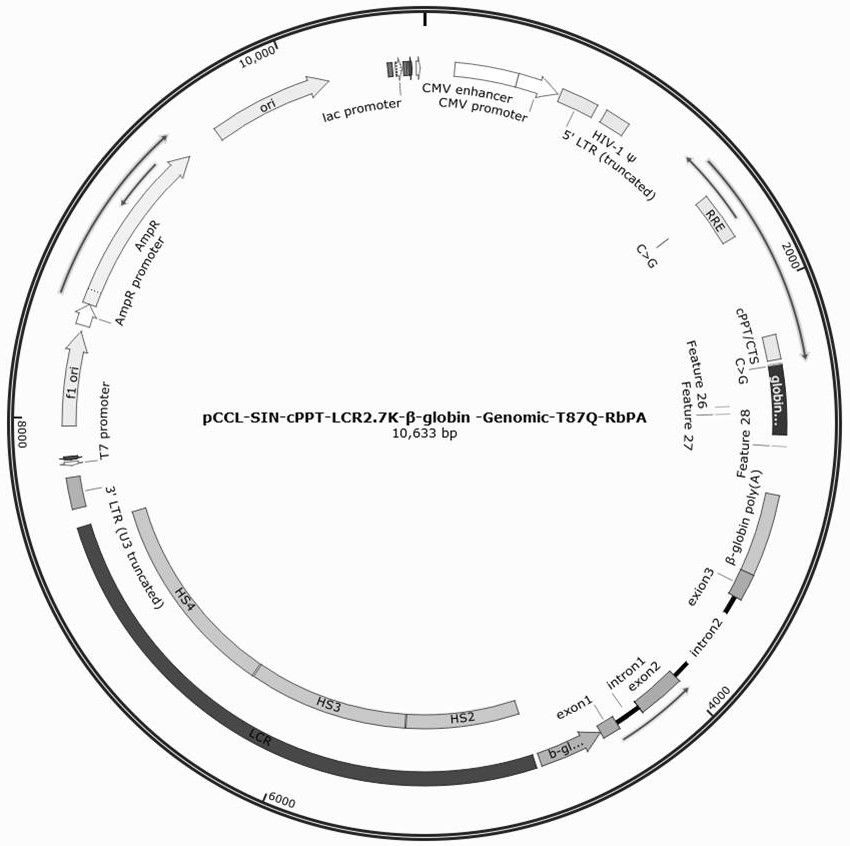
[0038] Example 1: Vector Construction
[0039]1. Construction of lentiviral vector pCCL-SIN-cPPT-LCR2.7K-β-globin-Genomic-T87Q-RbPA, including vector pCCL-SIN-cPPT-MCS-RbPA (SEQ ID No: 1), 2.7kb β- LCR regulatory sequence (SEQ ID No:2), β-globin promoter sequence (SEQ ID No:3), genome sequence encoding β-globin (SEQ ID No:4), β-globin polyA sequence (SEQ ID No: 5) and β-globin enhancer sequence (SEQ ID No: 6).
[0040] 1.1 Double-enzyme digestion of the pBSK vector plasmid with restriction enzymes ClaI and XhoI at 36.5-37.5°C for 0.8-1.2h, and after agarose electrophoresis, cut the gel to recover the pBSK vector fragment;
[0041] will express Hbβ A-T87Q The genomic sequence of the expression cassette is divided into β-globin1 and β-globin2 and carry out PCR respectively, wherein the primer sequence PCR1 used for the β-globin1 gene fragment is SEQ ID No:7, PCR2 is SEQ ID No:8, and the β-globin2 gene The primer sequences PCR3 used in the fragment are SEQ ID No: 9, and PCR4 i...
Embodiment 2
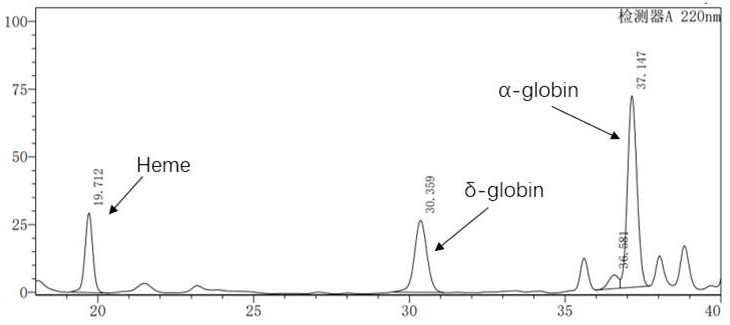
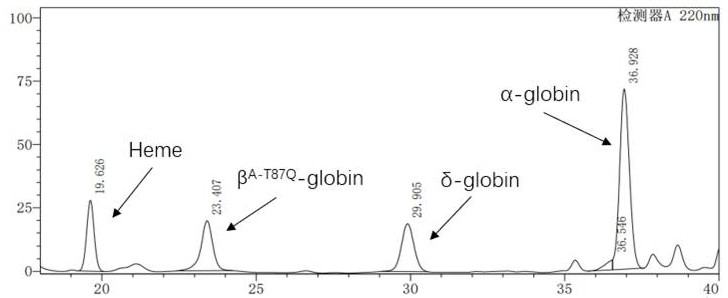
[0075] Example 2: Production and purification of lentivirus
[0076]2.1 The successfully constructed pCCL-SIN-cPPT-LCR2.7K-β-globin-Genomic-T87Q-RbPA plasmid and the lentiviral packaging kit plasmid Mix were co-transfected in a ratio of 1:3 and inoculated in a 10-layer cell factory one day before The HEK293T cells were replaced with fresh DMEM medium 6 hours after transfection, and the supernatant was collected after 72 hours for chromatographic purification;
[0077] 2.2 Purification Using a tangential flow filtration-chromatography system, using core700 chromatography and Q ImpRes chromatography purification process, the lentivirus was purified. The specific purification process is as follows:
[0078] ① Benzonase treatment (nuclease digestion): Treat with 25U / mL of Benzonase at 37°C for 1 hour to remove contaminants such as plasmid DNA remaining during transfection and genome released from lysed cells;
[0079] ② MF (clarification): The virus harvest solution is filtered (...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com