Torasemide genotoxic impurity detection method
A detection method, the technology of torasemide, applied in the field of chemical analysis, achieves high sensitivity, good specificity and repeatability, and accurate and reliable inspection results
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
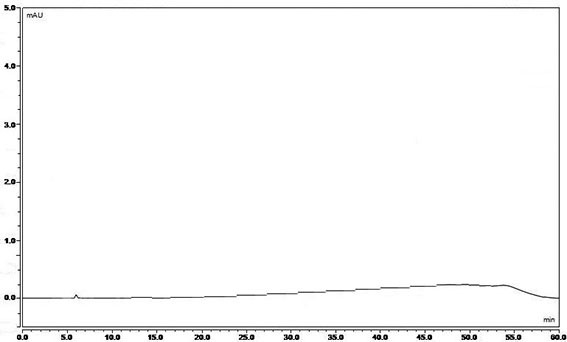
[0070] Chromatographic conditions: use octadecylsilane bonded silica gel as a filler or a column with equivalent performance
[0071] Mobile phase A: use 0.02mol / L disodium hydrogen phosphate buffer solution, adjust the pH value to 3.5 with 2mol / L phosphoric acid)
[0072] Mobile Phase B: Acetonitrile
[0073] Column temperature 35°C
[0074] Injection volume: 10ul
[0075] Flow rate: 0.8ml / min
[0076] Detection wavelength: 210nm
[0077] Solvent: 0.04% phosphoric acid solution-acetonitrile (80:20)
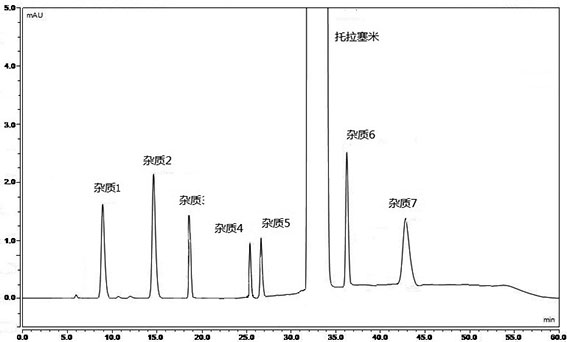
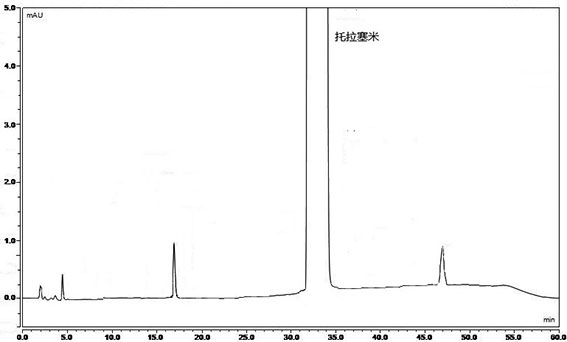
[0078] Mixed reference substance solution: Accurately weigh impurity 1-7 reference substance and torasemide reference substance, add solvent to dissolve and dilute to make a mixed reference substance solution containing 0.3 μg of each impurity and 20 mg of torasemide per 1 ml .
[0079] Need testing solution: take an appropriate amount of torasemide, add a solvent to dissolve and dilute to make a solution containing 20 mg of torasemide in every 1 ml.
[0080] Gradient elut...
Embodiment 2
[0083] The detection method is:
[0084] Chromatographic conditions: use octadecylsilane bonded silica gel as a filler or a column with equivalent performance
[0085] Mobile phase A: use 0.02mol / L disodium hydrogen phosphate buffer solution, adjust the pH value to 3.5 with 2mol / L phosphoric acid)
[0086] Column temperature 35°C
[0087] Injection volume: 10ul
[0088] Flow rate: 0.8ml / min
[0089] Detection wavelength: 210nm
[0090] Solvent: 0.03% phosphoric acid solution-acetonitrile (80:20)
[0091] Mixed reference substance solution: Accurately weigh impurity 1-7 reference substance and torasemide reference substance, add solvent to dissolve and dilute to make a mixed reference substance solution containing 0.3 μg of each impurity and 20 mg of torasemide per 1 ml .
[0092] Need testing solution: take an appropriate amount of torasemide, add a solvent to dissolve and dilute to make a solution containing 20 mg of torasemide in every 1 ml.
[0093] Gradient elution ...
Embodiment 3
[0096] The detection method is:
[0097] Chromatographic conditions: use octadecylsilane bonded silica gel as a filler or a column with equivalent performance
[0098] Mobile phase A: use 0.02mol / L disodium hydrogen phosphate buffer solution, adjust the pH value to 3.5 with 2mol / L phosphoric acid)
[0099] Mobile Phase B: Acetonitrile
[0100] Column temperature 35°C
[0101] Injection volume: 10ul
[0102] Flow rate: 0.8ml / min
[0103] Detection wavelength: 210nm
[0104] Solvent: 0.05% phosphoric acid solution-acetonitrile (80:20)
[0105] Mixed reference substance solution: Accurately weigh impurity 1-7 reference substance and torasemide reference substance, add solvent to dissolve and dilute to make a mixed reference substance solution containing 0.3 μg of each impurity and 20 mg of torasemide per 1 ml .
[0106] Need testing solution: take an appropriate amount of torasemide, add a solvent to dissolve and dilute to make a solution containing 20 mg of torasemide in ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com