Application of lentiviral vector in preparation of medicine for treating beta-thalassemia
A lentiviral vector and thalassemia technology, applied in the field of genetic engineering, can solve the problems of low titer of toxin production, bulky structure, high production cost, etc., and achieve the effect of increasing virus production, improving expression efficiency, and reducing the risk of cancer
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
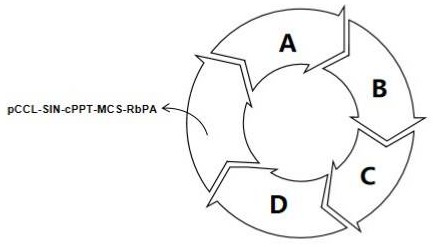
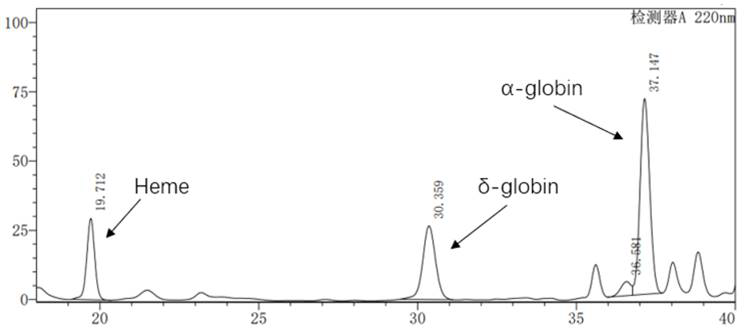
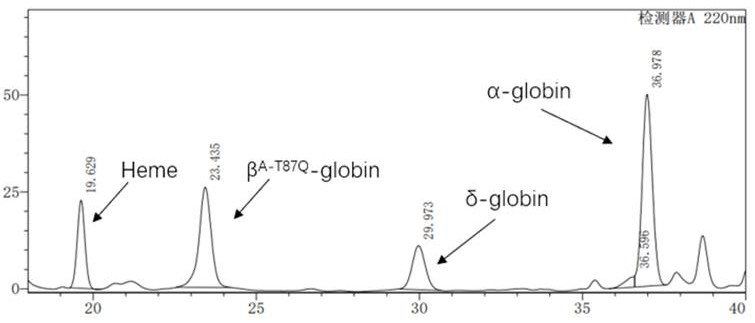
[0030] 1. Lentiviral vector pCCL-SIN-cPPT-LCR2.7K-pHBB-CI-β A-T87Q- Construction of RbPA, including vector pCCL-SIN-cPPT-MCS-RbPA (SEQ ID No: 1), 2.7kb β-LCR (LCR2.7K) regulatory sequence (SEQ ID No: 2), β-globin promoter ( pHBB) sequence (SEQ ID No:6), chimeric intron (CI) enhancer sequence (SEQ IDNo:7) and encoding codon-optimized β-globin gene β A-T87Q Sequence (SEQ ID No: 9), and the code at codon 87 contains the mutation T87Q, that is, the mutation from threonine to glutamine.
[0031] 1.1 The company's vector plasmid pCCL-SIN-cPPT-MCS-RbPA (SEQ ID No: 1) was used as the vector for the preparation of lentiviral vector, and it was double digested with restriction endonucleases XhoI and MluI at 36.5-37.5°C 0.8-1.2h, after agarose electrophoresis, the pCCL-SIN-cPPT-MCS-RbPA skeleton fragment was recovered by cutting the gel;
[0032] The LCR2.7K regulatory sequence (SEQ ID No: 2) was amplified by PCR, and the 5' end was added with a protection base and an XhoI restriction ...
Embodiment 2
[0101] 1. Lentiviral vector pCCL-SIN-cPPT-LCR3.6K-pHBB-CI-β A-T87Q - Construction of RbPA, including vector pCCL-SIN-cPPT-MCS-RbPA (SEQ ID No: 1), 3.6kb β-LCR (LCR3.6K) regulatory sequence (SEQ ID No: 3), β-globin promoter ( pHBB) sequence (SEQ ID No:6), chimeric intron (CI) enhancer sequence (SEQ IDNo:7) and encoding codon-optimized β-globin gene β A-T87Q Sequence (SEQ ID No: 9), and the code at codon 87 contains the mutation T87Q, that is, the mutation from threonine to glutamine.
[0102] 1.1 The company's vector plasmid pCCL-SIN-cPPT-MCS-RbPA (SEQ ID No: 1) was used as the vector for the preparation of lentiviral vector, and it was double digested with restriction endonucleases XhoI and MluI at 36.5-37.5°C 0.8-1.2h, after agarose electrophoresis, the pCCL-SIN-cPPT-MCS-RbPA skeleton fragment was recovered by cutting the gel;
[0103] The LCR3.6K regulatory sequence (SEQ ID No: 3) was amplified by PCR, and the 5' end was added with a protection base and an XhoI restriction...
Embodiment 3
[0131] 1. Lentiviral vector pCCL-SIN-cPPT-LCR4.6K-pHBB-CI-β A-T87Q - Construction of RbPA, including vector pCCL-SIN-cPPT-MCS-RbPA (SEQ ID No: 1), 4.6kb β-LCR (LCR4.6K) regulatory sequence (SEQ ID No: 4), β-globin promoter ( pHBB) sequence (SEQ ID No:6), chimeric intron (CI) enhancer sequence (SEQ IDNo:7) and encoding codon-optimized β-globin gene β A-T87Q Sequence (SEQ ID No: 9), and the code at codon 87 contains the mutation T87Q, that is, the mutation from threonine to glutamine.
[0132] 1.1 The company's vector plasmid pCCL-SIN-cPPT-MCS-RbPA (SEQ ID No: 1) was used as the vector for the preparation of lentiviral vector, and it was double digested with restriction endonucleases XhoI and MluI at 36.5-37.5°C 0.8-1.2h, after agarose electrophoresis, the pCCL-SIN-cPPT-MCS-RbPA skeleton fragment was recovered by cutting the gel;
[0133] The LCR4.6K regulatory sequence (SEQ ID No: 4) was amplified by PCR, and the 5' end was added with a protection base and an XhoI restriction...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com