Preparation method of 6-aminocaproic acid
A technology of aminocaproic acid and organic acid, which is applied in the field of preparation of 6-aminocaproic acid, can solve the problems of high energy consumption, cumbersome operation, unfavorable large-scale industrial production, etc., and achieve the effect of high content, simple purification and easy acquisition
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] A kind of preparation method of 6-aminocaproic acid
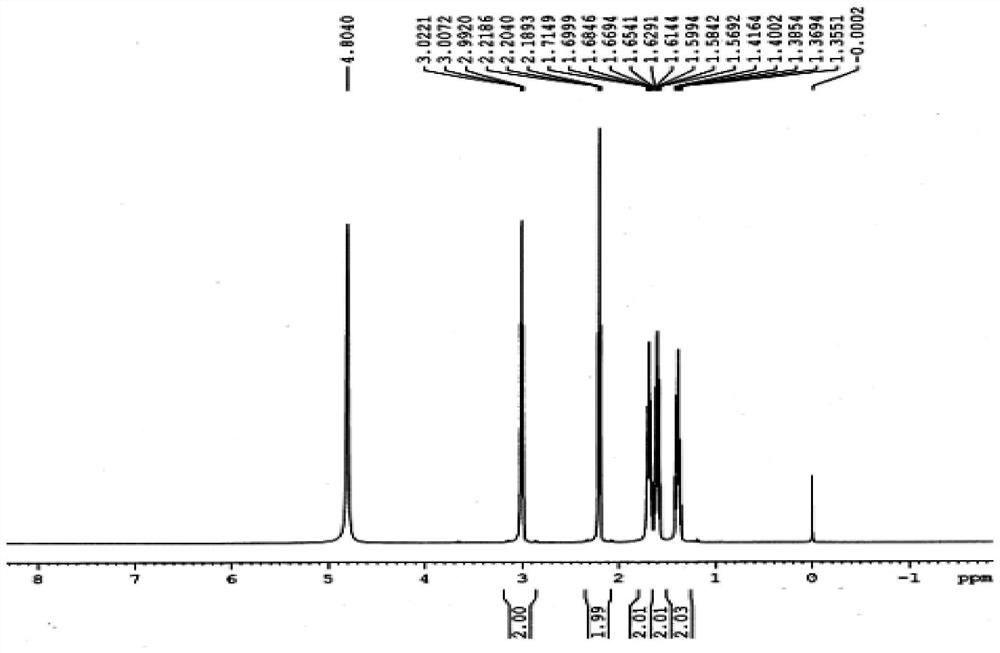
[0038] Mix 100g of caprolactam (0.88mol), 73.9g of potassium hydroxide (1.32mol) and 266g of water evenly, raise the temperature to 90°C, and react for 3h. Cool to 25°C, neutralize with 79.2g acetic acid (1.32mol), concentrate to dryness, add 598g ethanol, stir at 50°C for 4h, grow crystals at 0°C for 2h, filter to obtain a crude product. Add 59g of water to the crude product, heat until the crude product dissolves, add 467g of ethanol, grow crystals at 0°C for 2 hours, filter, and dry to obtain 89.5g of 6-aminocaproic acid with a purity of 99.8%, a content of 99.7%, and a mass yield of 89.5%. The theoretical yield is 115.4g, and the converted yield is 77.4%.
Embodiment 2
[0040] A kind of preparation method of 6-aminocaproic acid
[0041] Mix 100g of caprolactam (0.88mol), 147.8g of potassium hydroxide (2.64mol) and 266g of water evenly, raise the temperature to 120°C, and react for 1h. Cool to 25°C, neutralize with 195.4g propionic acid (2.64mol), concentrate to dryness, add 620g isopropanol, stir at 20°C for 4h, grow crystals at 5°C for 2h, filter to obtain a crude product. Add 59g of water to the crude product, heat until the crude product dissolves, add 480g of isopropanol, grow crystals at 5°C for 2h, filter, and dry to obtain 90.0g of 6-aminocaproic acid with a purity of 99.8%, a content of 99.7%, and a mass yield of 90.0 %. The theoretical yield is 115.4g, and the converted yield is 77.8%.
Embodiment 3
[0043] A kind of preparation method of 6-aminocaproic acid
[0044] Mix 100g of caprolactam (0.88mol), 147.8g of potassium hydroxide (2.64mol) and 500g of water evenly, raise the temperature to 105°C, and react for 6h. Cool to 25°C, neutralize with 380.7g isooctanoic acid (2.64mol), concentrate to dryness, add 580g n-propanol, stir at 80°C for 4h, grow crystals at 3°C for 2h, filter to obtain a crude product. Add 59g of water to the crude product, heat until the crude product dissolves, add 480g of n-propanol, grow crystals at 3°C for 2h, filter, and dry to obtain 90.2g of 6-aminocaproic acid with a purity of 99.8%, a content of 100.0%, and a mass yield of 90.2 %. The theoretical yield is 115.4g, and the converted yield is 78.2%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com