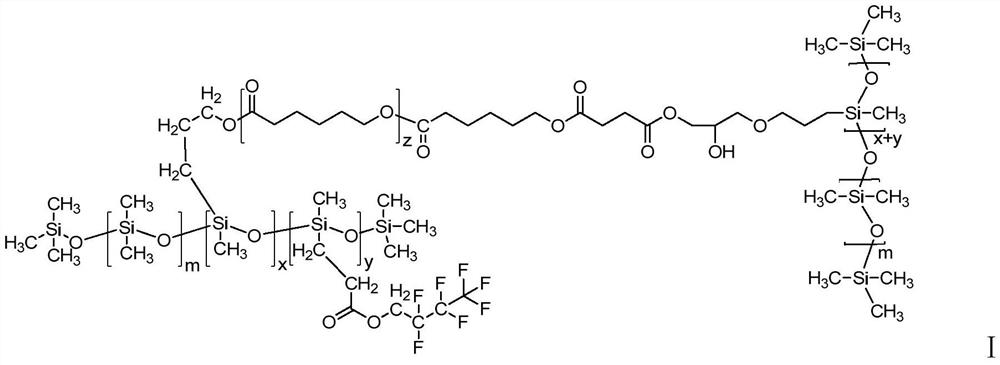
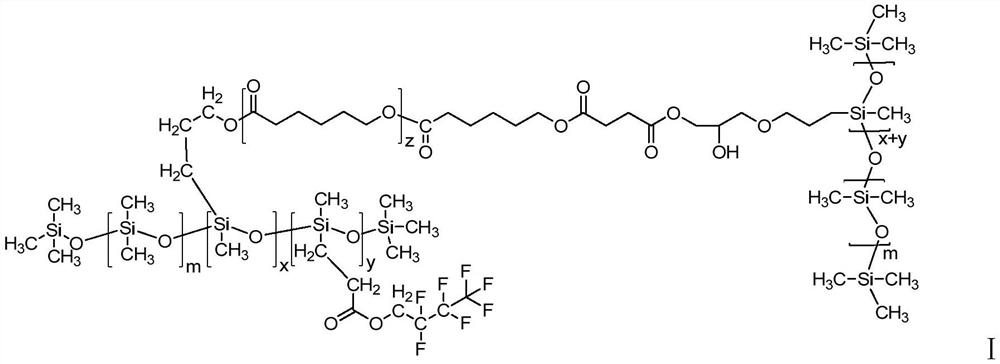
High-temperature-resistant fluorocarbon modified organic silicon resin as well as preparation method and application thereof
A fluorocarbon modification, silicone technology, used in coatings, polyester coatings, anti-corrosion coatings, etc., to achieve excellent high temperature resistance, improve hydrophobicity, and enhance impact resistance.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] Add 2664g of D4H, 5760g of D4, and 972g of hexamethyldisiloxane into the reactor, add 94g of concentrated sulfuric acid as a catalyst, and continue the reaction at 35°C until the D4 content in the system is less than 10%.
[0041] Distill the unreacted D4 in the system, add 469g of sodium bicarbonate to remove the remaining acid value until the acid value is less than 0.3mgKOH / g, filter and remove the solid to obtain intermediate A1, divide intermediate A1 into two parts, and mark them as Aa1, Ab1;
[0042] Add 1270g of heptafluorobutyl acrylate to 3100g of intermediate Aa1, add 200ppm of platinum catalyst after raising the temperature to 70°C, add 174g of allyl alcohol after a period of reaction, and continue the reaction until the infrared spectrum detects that the Si-H bond is complete After disappearing, add 684g of caprolactone and react at 130°C until the caprolactone is detected by HPLC After the reaction is complete, add 300g of succinic anhydride and react at 1...
Embodiment 2
[0046] Add 2880g of D4H, 12432g of D4, and 972g of hexamethylsiloxane into the system, add 162g of concentrated sulfuric acid as a catalyst, and continue the reaction at 35°C until the D4 content in the system is less than 10%.
[0047] Distill the unreacted D4 in the system, add 814g of sodium bicarbonate to remove the remaining acid value until the acid value is less than 0.3mgKOH / g, filter and remove the solid to obtain intermediate A2, divide intermediate A2 into two parts, and mark them as Aa2, Ab2;
[0048] Add 762g of heptafluorobutyl acrylate to 5428g of intermediate Aa2, add 200ppm of platinum catalyst after heating up to 70°C, add 58g of allyl alcohol after a period of reaction, and continue the reaction until the infrared spectrum detects that the Si-H bond is complete After disappearing, add 114g of caprolactone and react at 130°C until HPLC detects that the reaction of caprolactone is complete, then add 100g of succinic anhydride and react at 110°C until the chang...
Embodiment 3
[0052] Add 2760g of D4H, 10104g of D4, and 972g of hexamethylsiloxane into the system, add 138g of concentrated sulfuric acid as a catalyst, and continue the reaction at 35°C until the D4 content in the system is less than 10%.
[0053] Distill the unreacted D4 in the system, add 692g of sodium bicarbonate to remove the remaining acid value until the acid value is less than 0.3mgKOH / g, filter and remove the solid to obtain intermediate A3, divide intermediate A3 into two parts, and mark them as Aa3, Ab3;
[0054] Add 762g of heptafluorobutyl acrylate to 4612g of intermediate Aa3, add 200ppm of platinum catalyst after heating up to 70°C, add 290g of allyl alcohol after a period of reaction, and continue the reaction until the Si-H bond completely disappears by infrared detection Finally, add 1140g of caprolactone and react at 130°C until HPLC detects that the reaction of caprolactone is complete, then add 500g of succinic anhydride and react at 110°C until the acid value change...
PUM
| Property | Measurement | Unit |
|---|---|---|
| acid value | aaaaa | aaaaa |
| acid value | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com