Substituted triazine compound as well as preparation method and application thereof
A technology for compounds and triazine derivatives, applied in the field of biomedicine, can solve the problems of boric acid sensitivity toxicity, the influence of detection reagents and temperature, and limited application, and achieve the effect of increasing water solubility and improving biological development prospects.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
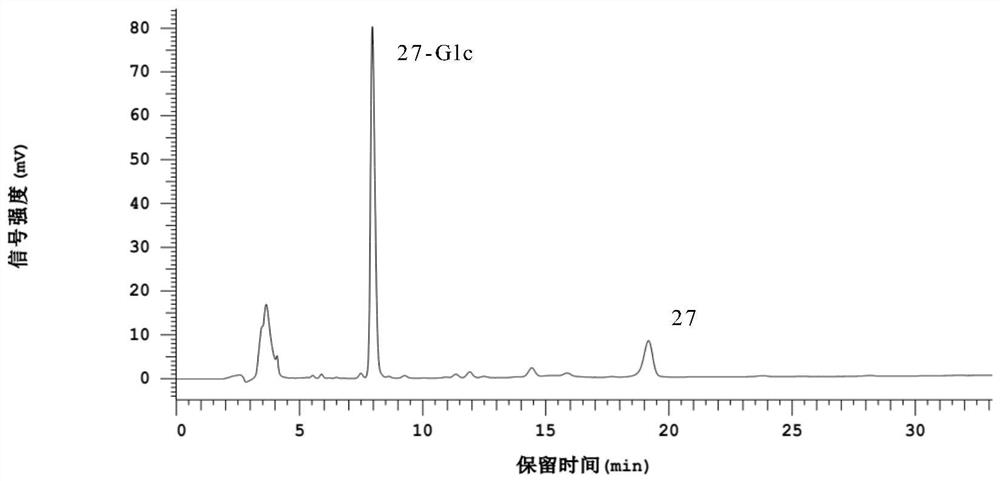
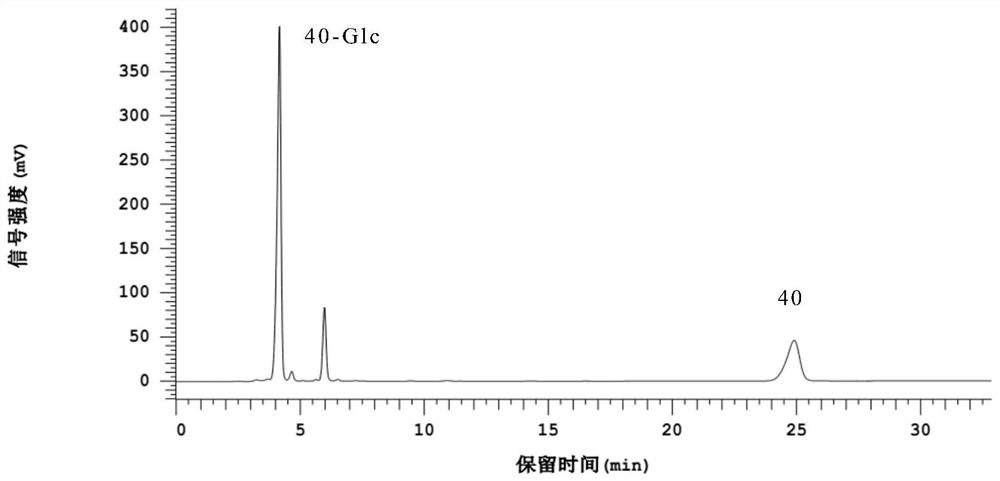
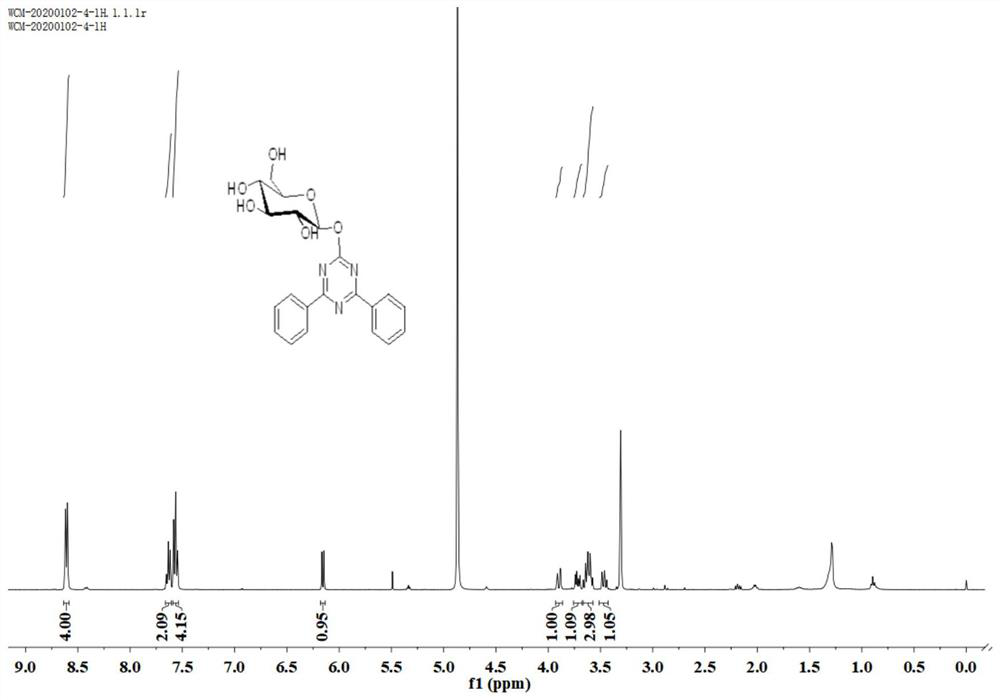
Image
Examples
Embodiment 1
[0050] In embodiment 1 structural formula (I), X 3 for O, R 3 For the preparation of alkylalkyne substituted triazine compounds and their quaternary ammonium salts:
[0051] Compound 1: 4-(4-(3-alkynyl-1-oxy)-1,3,5-triazin-2-yl)-4-methylmorpholin-4-amine hydrochloride
[0052] 2,4-Dichloro-1,3,5-triazine (500 mg, 3.4 mmol) was added to the reaction flask, 3-butyn-1-ol (5 mL) was added, and N,N-diisopropyl was slowly added dropwise Ethyl ethylamine (665 μL, 4.1 mmol), the solution changed from colorless to yellow and then red, and the reaction was stirred at room temperature for 30 min. It was extracted three times with ethyl acetate and saturated brine, and dried over anhydrous sodium sulfate. 500 mg was obtained by silica gel column chromatography, and the yield was 80%. MS(ESI): m / z, 250.1[M+H] + ; 1 H NMR (400MHz, Chloroform-d) δ8.74(s, 1H), 4.59(t, J=7.0Hz, 2H), 2.74(td, J=6.9, 2.7Hz, 2H), 2.05(t, J= 2.7Hz, 1H).
[0053] Add 2-(3-alkynyl-1-oxy)-4-chloro-1,3,5-triaz...
Embodiment 2
[0063] In embodiment 2 structural formula (I), X 3 for O, R 3 For the preparation of m-tolyl-substituted triazine compounds and their quaternary ammonium salts:
[0064] Compound 9: 2-Chloro-4-(m-tolyloxy)-1,3,5-triazine
[0065] 2,4-dichloro-1,3,5-triazine (500 mg, 3.4 mmol) and 5 mL of THF were added to the reaction flask for complete dissolution, and the mixture was stirred in an ice bath at 0°C. m-cresol (356 μL, 3.4 mmol) and DIPEA (665 μL, 4.1 mmol) were dissolved in 4 mL of THF, slowly added dropwise to the above reaction solution (the dropwise addition was completed in about 30 min), and the reaction was stirred at 0° C. for 30 min. It was extracted three times with ethyl acetate and saturated brine, and dried over anhydrous sodium sulfate. 590 mg was obtained by silica gel column chromatography, and the yield was 78.4%. MS(ESI): m / z, 222.0[M+H] + ; 1 H NMR (400MHz, Chloroform-d) δ 9.17 (s, 1H), 7.22 (t, J=7.5Hz, 1H), 7.05 (dt, J=7.5, 2.0Hz, 1H), 6.93 (dq, J= 8....
Embodiment 3
[0069] In embodiment 3 structural formula (I), X 3 for O, R 3 For the preparation of phenyl-substituted triazine compounds and their quaternary ammonium salts: the m-cresol used in the synthesis step of compound 9 is replaced with phenol, and according to the synthesis step of the second step of compound 1, N-methylmorpholine and The quaternary ammonium hydrochloride of pyridine can give compounds 12 and 13.
[0070] Compound 12: 4-methyl-4-(4-phenoxy-1,3,5-triazin-2-yl)morpholin-4-amine hydrochloride, 79% yield. MS(ESI): m / z, 274.1[M+H] + ; 1 H NMR (400MHz, Methanol-d 4 )δ9.10(s,1H),7.21(t,J=7.5Hz,2H),7.03-6.92(m,3H),4.48(ddd,J=12.0,10.3,7.0Hz,2H),4.43-4.33 (m, 2H), 4.21–4.11 (m, 2H), 4.05 (ddd, J=12.2, 6.3, 1.2Hz, 2H), 3.25 (s, 3H). 13 C NMR (101MHz, Methanol-d 4 ) δ177.14, 162.90, 160.20, 152.29, 129.39, 124.49, 121.64, 66.27, 49.68, 40.55.
[0071] Compound 13: 1-(4-phenoxy-1,3,5-triazin-2-yl)pyridin-1-amine hydrochloride in 69% yield. MS(ESI): m / z, 252.1[M+H] + ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com