Blood brain barrier high-permeability hexokinase inhibitor as well as synthesis method and application thereof
A technology of compounds and hydrates, applied in the directions of organic active ingredients, chemical instruments and methods, active ingredients of heterocyclic compounds, etc., to achieve the effect of improving the permeability of the blood-brain barrier
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
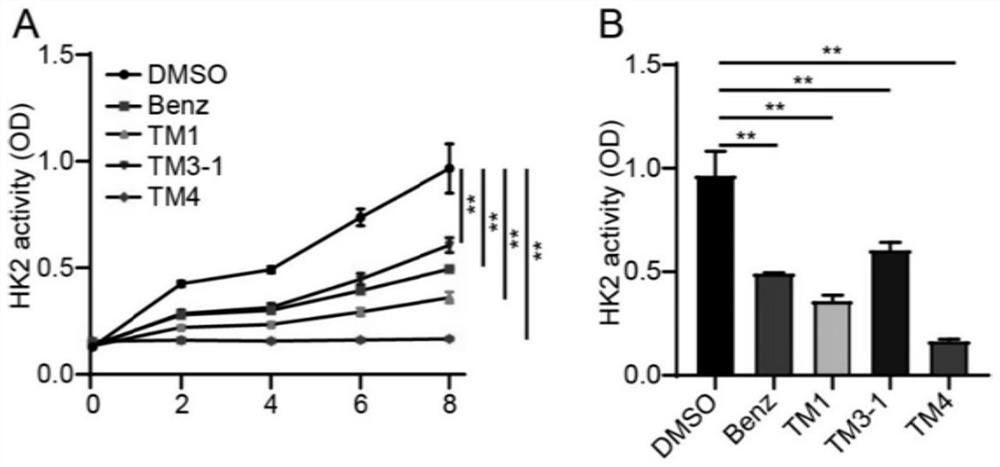
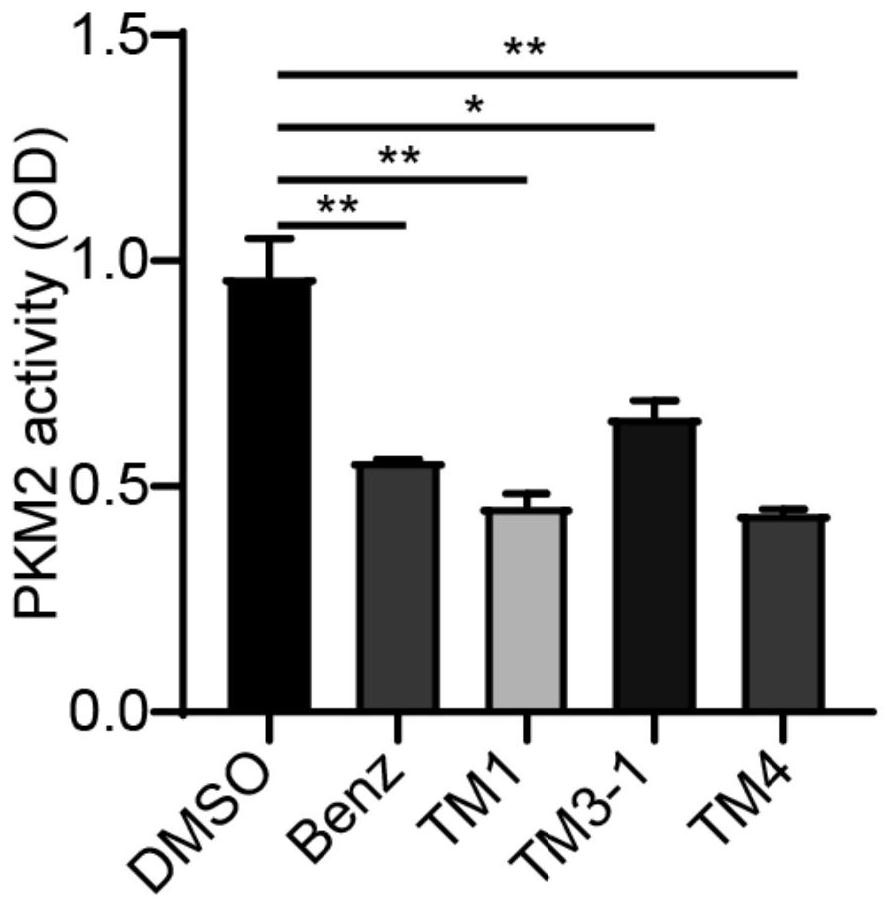
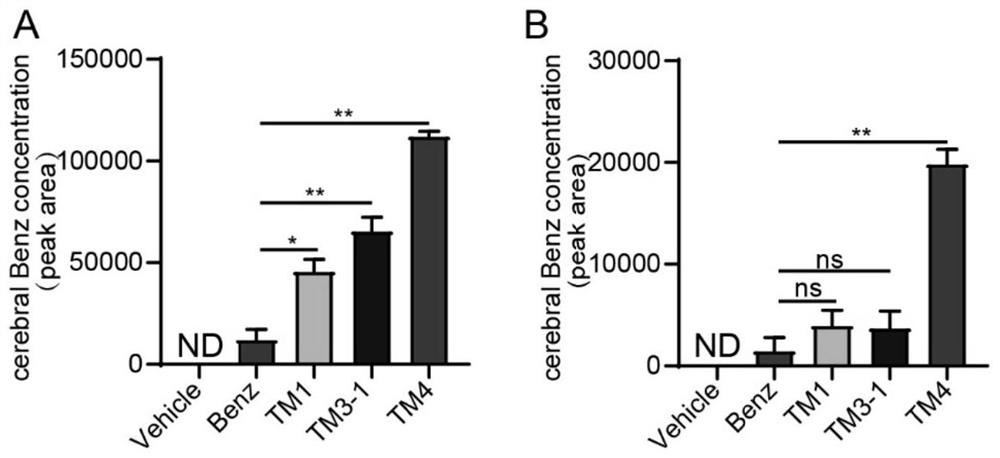
Image
Examples
preparation example Construction
[0098] Specifically, the synthetic method of the present invention comprises:
[0099] The serine methyl ester hydrochloride is reacted with hydrazine hydrate in an alcohol solvent to obtain the amine transesterified compound IM1 serine hydrazide hydrochloride. The reaction time is 12-72h, preferably 24-72h.
[0100] In an inert solvent, the 3,4-position phenolic hydroxyl groups on compound S4 and S3 are ketalized under the catalyst of acid to form a ring to form compound IM3, and then the 2-position phenolic hydroxyl group in compound IM3 is reacted with acid chloride or its analogs. The esterification reaction is carried out to obtain compound IM4 in which all three hydroxyl groups at positions 2, 3, and 4 are protected.
[0101] Compound IM4 and IM1 undergo condensation reaction in alcohol solvent to prepare compound of formula II.
[0102] The compound of formula II undergoes a hydrogenation reaction under the action of a catalyst and hydrogen to obtain a benserazide der...
Embodiment 1
[0133] The synthetic route of the present embodiment is as follows:
[0134]
[0135] Preparation of compound IM1: react serine methyl ester hydrochloride and hydrazine hydrate in methanol solvent for 2 days, and a white solid is precipitated to obtain compound IM1, 1 H NMR (400MHz, D2O) δ3.83 (dt, J=5.6, 4.1Hz, 1H), 3.74–3.73 (m, 1H), 3.71–3.70 (m, 1H), 3.68 (d, J=0.6Hz, 1H), 3.67(d, J=0.6Hz, 1H), 3.65(d, J=0.6Hz, 1H), 3.64(d, J=0.6Hz, 1H).
[0136] Preparation of compound IM2: using trimethyl orthoacetate (80 mmol) as a solvent, p-toluenesulfonic acid monohydrate (4 mmol) as a catalyst, 2,3,4 trihydroxybenzaldehyde (40 mmol) and trimethyl orthoacetate at 110 The reaction was refluxed in a round-bottomed flask at ℃ for 2 days, and a solid was precipitated. The product was subjected to suction filtration, and washed with the filtrate to obtain a white solid, weighing 3.18 g, and the yield was 38% to obtain compound IM2, 1 H NMR (400MHz, CHLOROFORM-D)δ11.03-10.89(m,1H),9.8...
Embodiment 2
[0141] The synthetic route of the present embodiment is as follows:
[0142]
[0143] Preparation of compound IM1: react serine methyl ester hydrochloride and hydrazine hydrate in methanol solvent for 2 days, and a white solid is precipitated to obtain compound IM1, 1 H NMR (400MHz, D2O) δ3.83 (dt, J=5.6, 4.1Hz, 1H), 3.74–3.73 (m, 1H), 3.71–3.70 (m, 1H), 3.68 (d, J=0.6Hz, 1H), 3.67(d, J=0.6Hz, 1H), 3.65(d, J=0.6Hz, 1H), 3.64(d, J=0.6Hz, 1H).
[0144] Compound IM2 was prepared by using trimethyl orthoacetate as a solvent and p-toluenesulfonic acid monohydrate (4 mmol) as a catalyst. The reaction was refluxed in the bottom flask for 2 days, and a solid was precipitated. The product was filtered with suction and washed with the filtrate to obtain a white solid, which weighed 3.18 g and the yield was 38% to obtain compound IM2, 1 H NMR (400MHz, CHLOROFORM-D)δ11.03-10.89(m,1H),9.80-9.68(m,1H),7.48-7.05(m,1H),6.64-6.43(m,1H),3.48-2.91 (m,3H),1.89–1.76(m,3H).
[0145] Preparat...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com