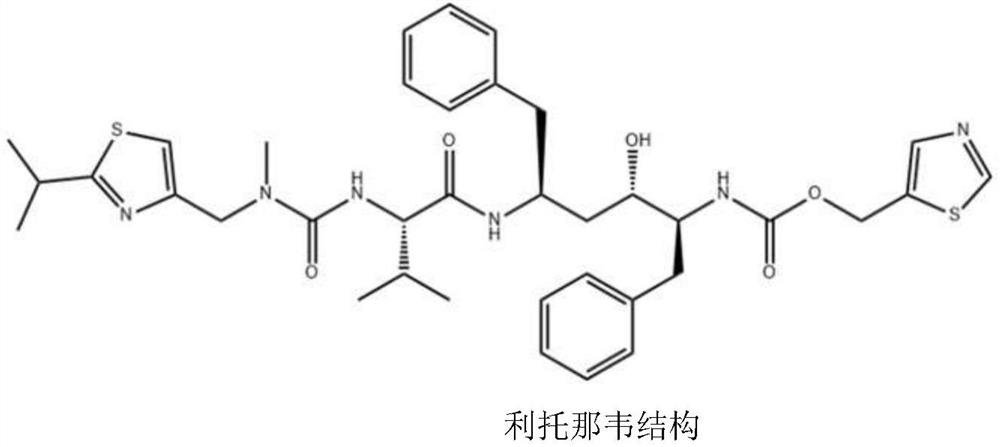
Preparation method of ritonavir solid dispersion
A technology of solid dispersion and ritonavir, which is applied in the directions of non-active ingredients medical preparations, medical preparations containing active ingredients, and pharmaceutical formulas, which can solve problems such as adverse reactions and ineffectiveness
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0019] Embodiment 1 screens different carrier types and prepares solid dispersion
[0020] experiment procedure:
[0021] (1) Ritonavir was mixed with polyethylene glycol 3000, polyvinylpyrrolidone K30, and copovidone at a ratio of 1:5, respectively, and then placed in an autoclave.
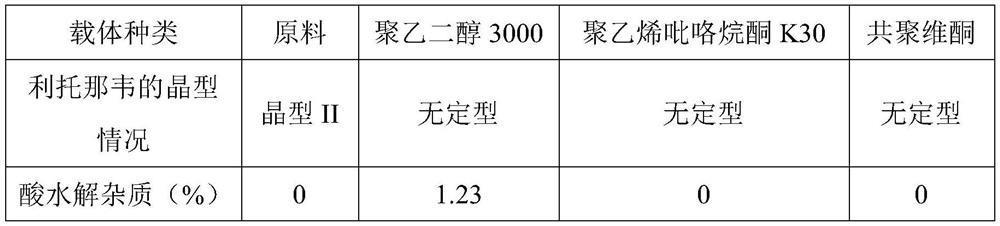
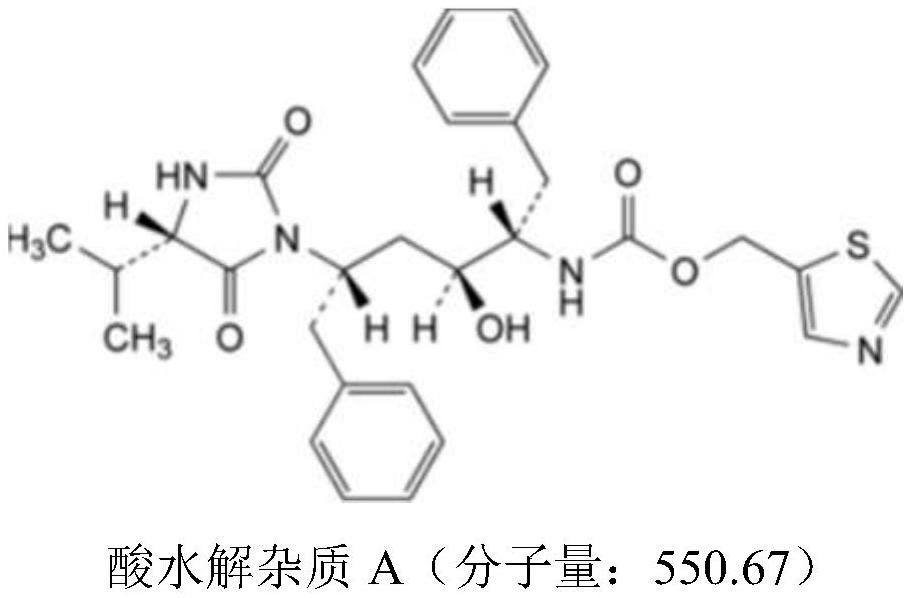
[0022] (2) The temperature in each kettle is set to 45°C, and carbon dioxide is introduced until the pressure reaches 25Mpa. After the preparation time is 5 hours, the solid dispersion is taken out, ground, and the crystal form is detected by X-ray diffraction method together with the raw materials, and the The HPLC method was used to detect the content of acid hydrolyzed impurities. The results showed that the solid dispersion prepared by polyethylene glycol 3000 and ritonavir had a large content of acid hydrolysis impurities, and polyvinylpyrrolidone K30 or copovidone could be used.
[0023] Table 1 adopts the crystal form and impurity situation of solid dispersion prepared by different carri...
Embodiment 2
[0036] Embodiment 2 investigates the different ratios of ritonavir and copovidone in carbon dioxide under the critical state
[0037] experiment procedure:
[0038] (1) Mix ritonavir and copovidone at a ratio of 1:1, 1:3, 1:5, 1:7, and 1:9, respectively, and place them in an autoclave.
[0039] (2) The temperature in each kettle was set to 45°C, and carbon dioxide was introduced until the pressure reached 25Mpa. After the preparation time was 5 hours, the solid dispersion was taken out, and the crystal form was detected by X-ray diffraction method after grinding.
[0040] (3) The test results show that when the ratio of ritonavir and copovidone is 1:5-1:9, ritonavir is amorphous in solid dispersion.
[0041] Table 2 prepares the crystal form of solid dispersion at different temperatures
[0042] Ratio of ritonavir and copovidone 1:1 1:3 1:5 1:7 1:9 Crystal form of ritonavir partially crystalline partially crystalline not finalized not finalized n...
Embodiment 3
[0043] Embodiment 3 screening critical carbon dioxide prepares the temperature of solid dispersion
[0044] experiment procedure:
[0045] (1) After mixing ritonavir and copovidone evenly according to the ratio of 1:5, divide them into 5 parts on average, and put 4 parts in the autoclave respectively.
[0046] (2) Set 35°C, 40°C, 45°C, and 50°C respectively, and feed carbon dioxide until the pressure reaches 25Mpa. After the preparation time is 5 hours, take out the solid dispersion, grind it, and use it together with the mixture before preparing the solid dispersion The crystal form was detected by X-ray diffraction method, and the acid hydrolysis impurity content was detected by HPLC method. Tests show that the set temperature for preparing the solid dispersion should be 40-50°C.
[0047] (3) Adopt the hot-melt extruder of different direction conical screw to prepare solid dispersion, after ritonavir and copovidone are mixed according to 1:5 ratio, screw speed is set to be...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com