Synthetic method of nitrogen-containing heterocyclic compound and intermediate thereof
A synthetic method and compound technology, applied in organic chemistry, bulk chemical production, etc., can solve the problems of lengthy synthetic method routes, unfavorable industrial scale-up production, high cost of raw materials and equipment, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0060] The present invention provides a method for synthesizing intermediates of nitrogen-containing heterocyclic compounds, comprising the steps of:
[0061] carrying out photochemical reaction of compound 1 and compound 2 to prepare the intermediate;
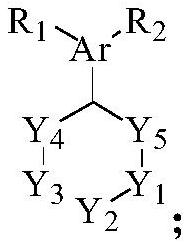
[0062] The structure of the compound 1 is as follows:
[0063]
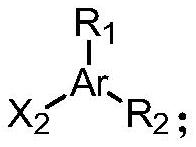
[0064] The structure of the compound 2 is as follows:
[0065]
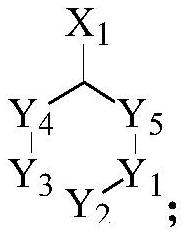
[0066] The structure of the intermediate is shown below:
[0067]
[0068] Among them, Y 1 , Y 2 , Y 3 , Y 4 , Y 5 Each independently is CH 2 or NR, and at least one is NR; R represents a protecting group;
[0069] x 1 、X 2 each independently is a halogen;
[0070] Ar is C6~C10 aryl or C5~C10 heteroaryl;
[0071] R 1 , R 2 each independently hydrogen, halogen, cyano, hydroxyl, C1-C6 alkoxy or -S(O) 2 R 3 , R 3 It is a C1-C6 alkyl group.
[0072] In one specific example, the light source used for the photochemical reaction is an 8W-12W blue light. Specifically, the power o...
Embodiment 1
[0120] The synthesis of embodiment one compound 3A
[0121]
[0122]Under nitrogen protection, 3-bromopiperidine-1-carboxylic acid tert-butyl ester (compound 1A) (896mg, 3.40mmol, 1.3eq), 4-bromochlorobenzene (compound 2A) (500mg, 2.61mmol, 1.00eq), [4,4′-bis(1,1-dimethylethyl)-2,2′-bipyridineN1,N1]bis[3,5-difluoro-2-[5-(trifluoromethyl) -2-pyridyl N]phenyl-C]iridium(III) hexafluorophosphate (29.3mg, 0.01eq), [4,4′-bis(1,1-dimethylethyl)-2,2′ - bipyridine N1, N1] nickel(II) chloride (15.6mg, 0.015eq), tris(trimethylsilyl) silane (650mg, 1.00eq), sodium carbonate (554mg, 2.00eq) and 26ml of Ethylene glycol dimethyl ether was added into a 40 ml reaction bottle, irradiated with a 10W blue LED light at a distance of 3 cm from the reaction bottle, and the mixture was stirred and reacted at 25° C. for 14 hours. After the reaction was monitored by LCMS, the mixture was filtered, and the filtrate was concentrated to obtain a crude product. The crude product was separated by prep...
Embodiment 2
[0126] The synthesis of embodiment two compound A1
[0127]
[0128] A mixture of compound 3A (20.0mg, 67.6μmol, 1.00eq) and hydrochloric acid / ethyl acetate (4M, 1mL) was stirred at 15°C for 2 hours. After the reaction was monitored by LCMS, the reaction solution was concentrated to obtain Compound A1 (15 mg, 64.3 μmol, 99.5% purity, 95.1% yield) as a white solid.
[0129] 1 H NMR: (400MHz, DMSO-d 6 )δ9.08(br s,1H),7.41(d,J=8.4Hz,2H),7.32(d,J=8.4Hz,2H),,3.30-3.25(m,3H),3.03-2.98(m ,2H), 2.89-2.83(m,1H),1.88-1.65(m,4H).
[0130] LC-MS: (M+H) + :196.2.
[0131] HPLC: 99.5% purity (220nm, Rt=2.306min).
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap