Monoclonal antibody specifically combined with ricin and application thereof
A ricin, specific technology, applied in the field of immunity, can solve the problem of high toxicity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
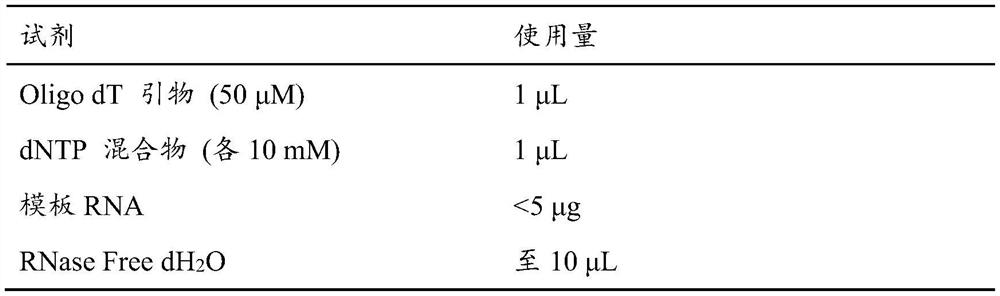
[0032] The extraction of embodiment 1.RT crude poison
[0033] Take 50g of castor bean and shell it, put it in a pulverizer, add liquid nitrogen to pulverize it, and take it out. Then add 100 mL of 10 mM phosphate buffer (pH 7.2), leaching at 4 °C for 24 h, centrifugation at 17000 g for 40 min at 4 °C, to remove the precipitate and supernatant, collect the supernatant and filter it with three layers of filter paper. Then add saturated ammonium sulfate solution, set at 4°C overnight, centrifuge at 17000g for 40min at 4°C, and collect the precipitate. The obtained precipitate was dissolved in 10 mM phosphate buffer (pH 7.2), and dialyzed with 10 mM phosphate buffer (pH 7.2) overnight, centrifuged at 17,000 g at 4°C for 15 min, and the supernatant obtained was crude ricin.
Embodiment 2
[0034] Example 2. Purification of RT crude virus
[0035] (1) Affinity chromatography
[0036] Add an appropriate amount of crude ricin into the balanced Sepharose 4B medium, mix and absorb it for 1.5 hours, put the medium into the gravity column, connect the constant flow pump and control the flow rate to 70r / min, and use the HD-A chromatogram to collect and analyze The chromatogram was recorded by the instrument. The impurity protein was washed with 10 mM phosphate buffer (pH 7.2), and after the UV curve was flattened, 150 mM galactose-PBS (pH 7.2) was added to elute the protein, and the elution peaks were collected.
[0037] (2) Molecular sieve chromatography
[0038] The collected solution after affinity was loaded onto a Sephacryl S-100HR prepacked column at a flow rate of 0.5 mL / min, eluted with 10 mM phosphate buffer (pH 7.2) buffer, and the elution peaks were collected.
Embodiment 3
[0039] Example 3. RT identification and protein concentration determination
[0040] The collected RT proteins were boiled with β-mercaptoethanol-containing and β-mercaptoethanol-free loading buffers, respectively, and run for SDS-PAGE electrophoresis. It was stained with Coomassie brilliant blue R-250, and the destaining solution was decolorized on a shaking table, and the relative molecular weights of RT, RTA and RTB were determined by analyzing the gel image. The concentration of RT was determined by the BCA method.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com