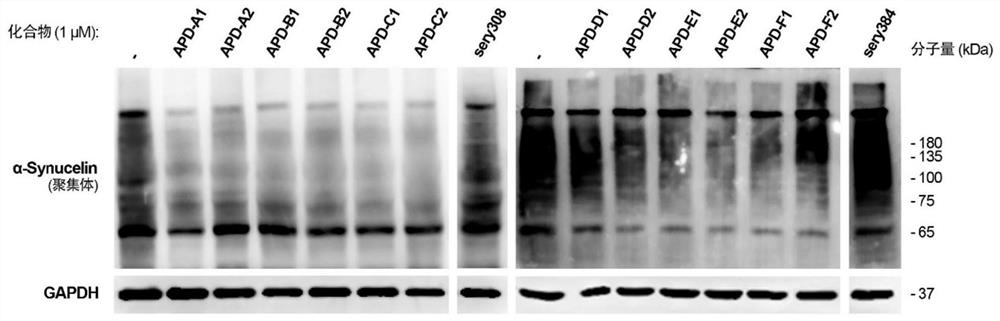
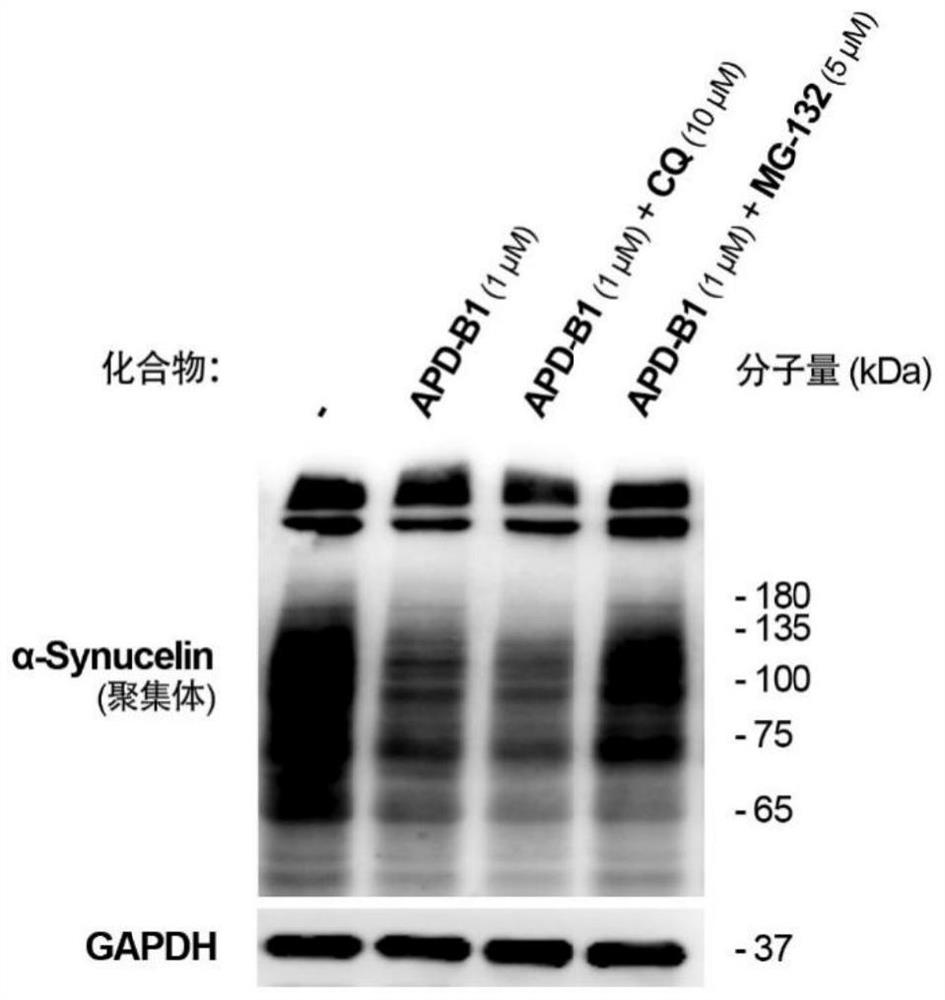
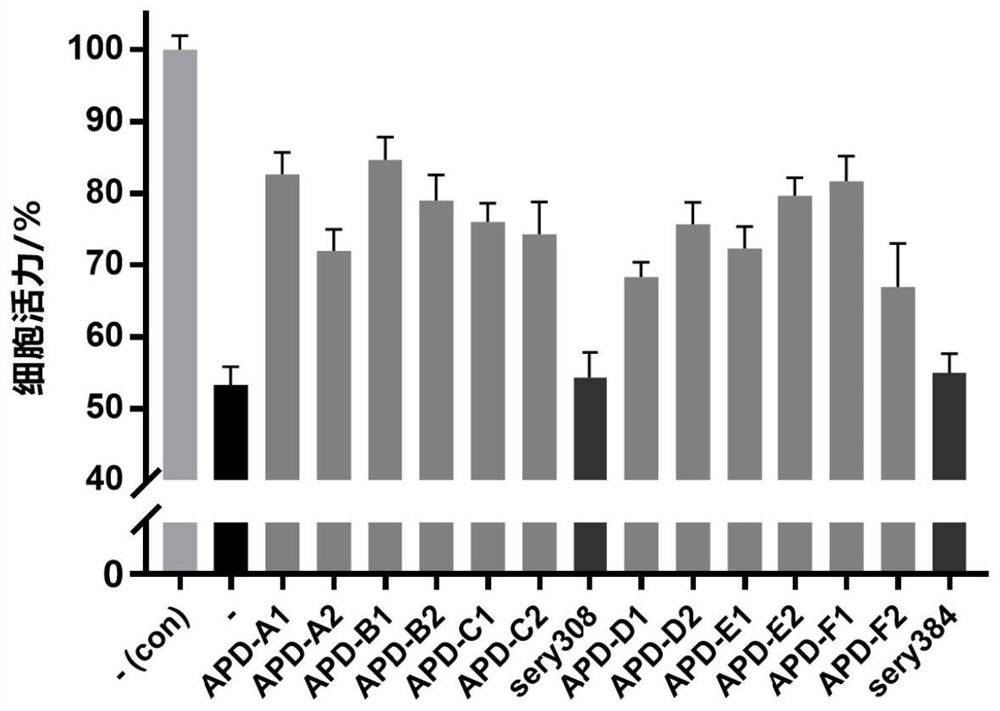
Alpha-synuclein targeting compound as well as preparation method and application thereof
A technology of synuclein and compound, which is applied to α-synuclein targeting compound and preparation method and application field thereof, can solve the problem of inability to directly degrade α-synuclein, etc. Apply foreground, lower level effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] Example 1 Synthesis and structure confirmation of APD-A series compounds
[0042] The synthetic route and condition of described APD-A series compound are as follows:
[0043]
[0044] (a) NaOH, Ba(OH) 2 ·8H 2 O, MeOH, RT, 24h; (b) H 2 O 2 , NaOH, MeOH, 0°C to RT, 24h; (c) NH 2 NH 2 ·H 2 O, p-TsOH, toluene, 90℃, 4h; (d) DHP, p-TsOH, THF, flux, 24h; (e) H 2 , Pd / C, RT, 24h; (f) (Boc 2 )O, Et 3 N, MeOH, flux, 3h; (g) p-TsCl, DMAP, DCM, RT, 1h+1h; (h) (Boc 2 )O, NaOH, H 2 O / acetone, RT, 24h; (i) DIEA, DMF, 90°C, 24h; (j) TFA, DCM, RT, 2h; (k) EDC·HCl, HOBT, DIEA, DMF, RT, 24h.
[0045] Specific methods and structure confirmation:
[0046] General method one (deprotection):
[0047] Dissolve about 1 mmol of the reactant in 10 mL of a mixed solvent of dichloromethane / trifluoroacetic acid (1:1), and react at room temperature for 2 h under vigorous stirring; after the reaction is completed, remove the solvent under reduced pressure for several times, and dry in...
Embodiment 2
[0089] Example 2 Synthesis and structure confirmation of APD-B series compounds
[0090] The synthetic route and condition of described APD-B series compound:
[0091]
[0092] (a) DIEA, DMF, 90℃, 24h; (b) TFA, DCM, RT, 2h; (c) EDC·HCl, HOBT, DIEA, DMF, RT, 24h.
[0093] Specific synthesis method and structure confirmation:
[0094] Synthesis of compound 18:
[0095] Dissolve 0.5 mmol of compound 7 (182 mg) and 1 mmol of N,N-diisopropylethylamine (164 μL) in 5 mL of N,N-dimethylformamide, then add 0.5 mmol of tert-butyl bromoacetate (compound 16, 75 μL ), reacted at room temperature or heated to 90 °C for 24 h; after the reaction was completed, cooled to room temperature, diluted with water, extracted with ethyl acetate, combined the organic phases, washed with water and brine respectively, and dried with anhydrous magnesium sulfate, the obtained crude product was subjected to flash column Purified by chromatography (petroleum ether / ethyl acetate, 2:1), and dried in vacu...
Embodiment 3
[0109] Example 3 Synthesis and structure confirmation of APD-C series compounds
[0110] The synthetic route and condition of described APD-C series compound:
[0111]
[0112] (a) CBr 4 , PPh 3 , DCM, 0°C to RT, 12h; (b) DIEA, DMF, 90°C, 24h; (c) TFA, DCM, RT, 2h; (d) EDC·HCl, HOBT, DIEA, DMF, RT, 24h.
[0113] Specific preparation method and structure confirmation:
[0114] Synthesis of compound 24a:
[0115] Dissolve 1 mmol of carboxyl tripolyethylene glycol tert-butyl ester (compound 23a, 234 mg) in 10 mL of dichloromethane, cool to 0 °C, and then add 1 mmol of carbon tetrabromide (331 mg) and 1 mmol of triphenylphosphine in portions under stirring (262mg), reacted at room temperature for 12h; after the reaction was completed, the solvent was removed under reduced pressure, resuspended with water, extracted with ethyl acetate, the organic phases were combined, washed with water and brine respectively, and dried with anhydrous magnesium sulfate. Purification by flas...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com