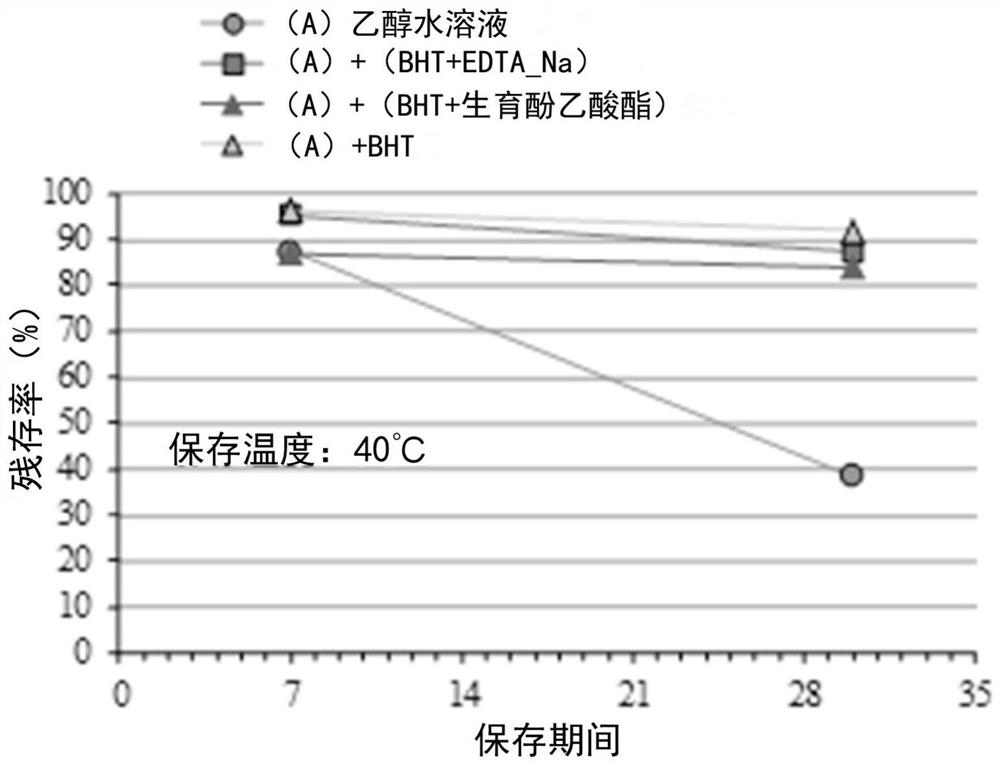
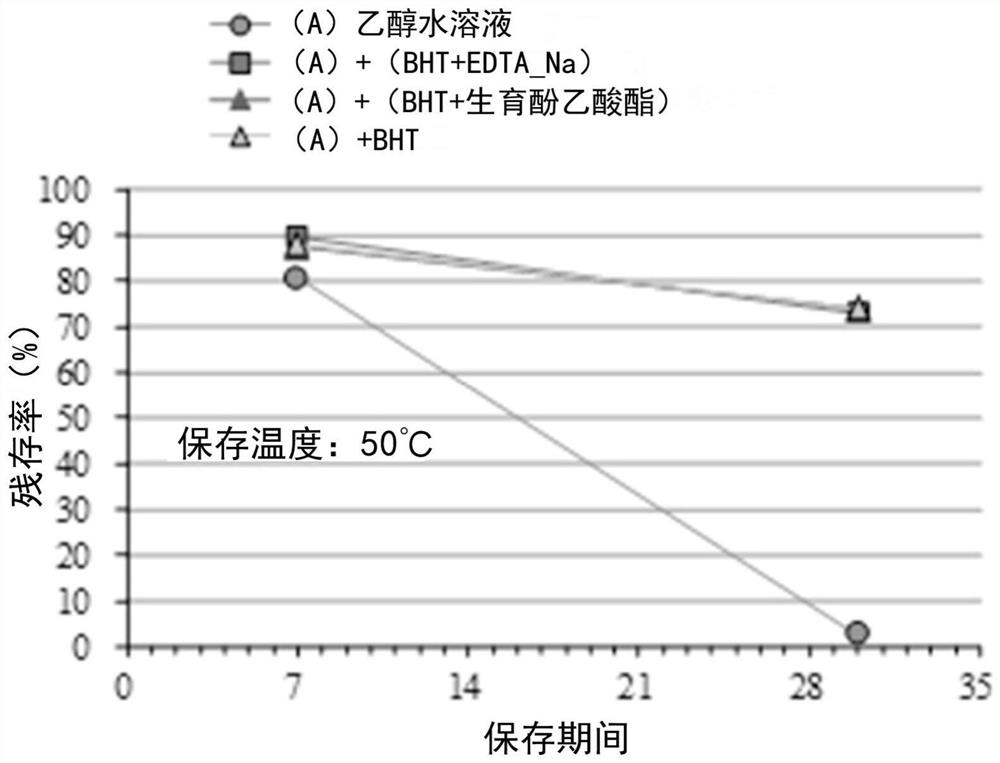
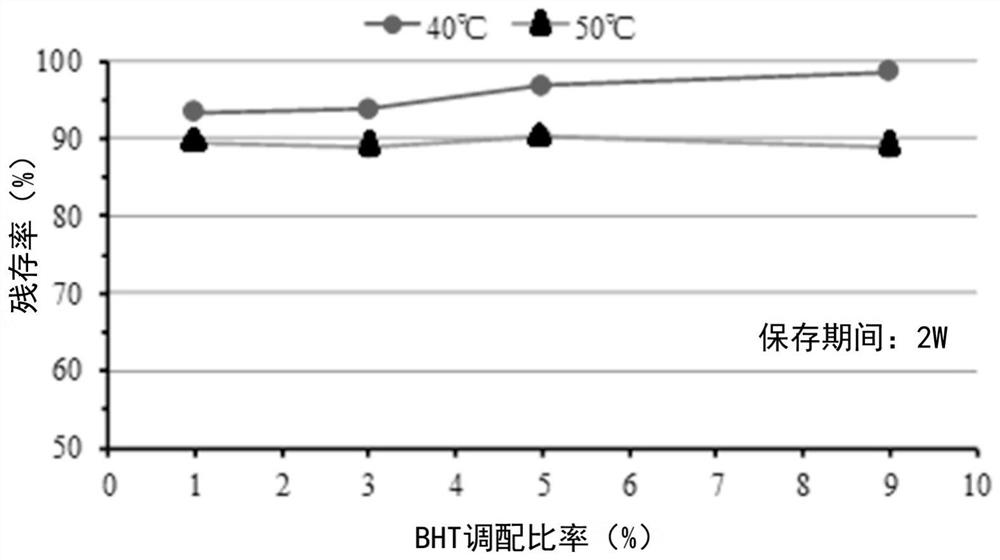
External preparation containing rapamycin
A technology of rapamycin and external preparations, which is applied in the directions of medical preparations containing active ingredients, medical preparations without active ingredients, and aerosol delivery, etc. Rapamycin deterioration and other problems
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0096] Hereinafter, the present invention will be described with reference to specific embodiments, but the present invention is not limited to the embodiments, and those skilled in the art will understand that the present invention may be used without departing from the scope or spirit of the present invention defined in the appended claims. Various changes and modifications are made in this embodiment below.
reference example 1
[0098] Sirolimus was mixed with each solvent shown in Table 1 in a ratio of sirolimus:solvent at 1:50 (weight ratio), heated in a water bath at about 50°C, irradiated with ultrasonic waves, and visually inspected. Observation confirmed the solubility. As a result, as shown in Table 1, it was not confirmed that sirolimus was dissolved in a single solvent.
[0099] [Table 1]
[0100] Solubility of sirolimus in each solvent
[0101] solvent Solubility Medium Chain Fatty Acid Triglycerides Insoluble (turbid liquid) Glycol Salicylate Insoluble (turbid liquid) Diethyl Sebacate Insoluble (turbid liquid) Diisopropyl Sebacate Insoluble (turbid liquid) Triacetin Insoluble (turbid liquid) Squalene Insoluble (turbid liquid) Propylene Glycol Insoluble (turbid liquid) Polyethylene Glycol (Macrogol 400) Insoluble (turbid liquid) 1,3-Butanediol Insoluble (turbid liquid)
[0102] (Quantitative test of sirolimus) ...
Embodiment 1
[0109] Example 1, Comparative Example 1
[0110] Using a mixed solvent obtained by mixing each solvent described in Table 1 and propylene glycol, the mixture was mixed so that sirolimus:solvent:propylene glycol was 1:50:25 (weight ratio), and heated in a water bath at about 50°C , and ultrasonic irradiation was carried out to prepare various preparations. Solubility was confirmed by visual observation of the obtained formulation.
[0111] The solubility results are shown in Table 2. The dissolution of sirolimus was confirmed by using a mixed solution of ethylene glycol salicylate, diethyl sebacate, and triacetin and propylene glycol.
[0112] [Table 2]
[0113] Solubility of Sirolimus to Mixtures of Each Solvent and Propylene Glycol (PG)
[0114] Test example solvent Solubility Comparative Example 1-1 Medium chain fatty acid triglycerides + PG Insoluble (white turbidity precipitates out) Example 1-1 Glycol Salicylate + PG dissolve Exa...
PUM
| Property | Measurement | Unit |
|---|---|---|
| The inside diameter of | aaaaa | aaaaa |
| Length | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com