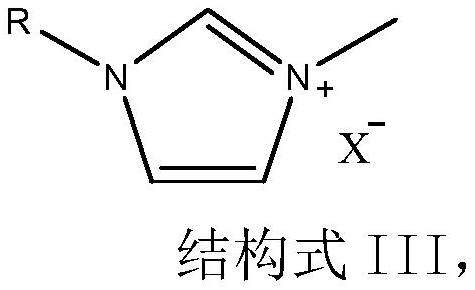
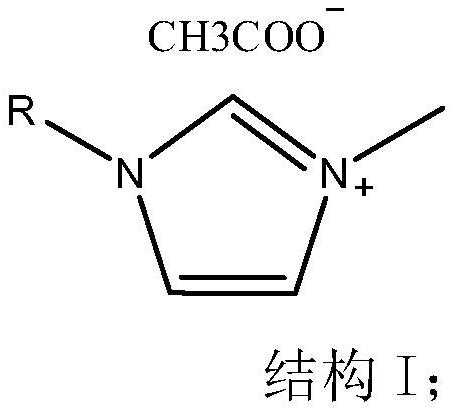
Method for efficiently preparing high-content 1, 3-dialkyl imidazole ionic liquid
A technology of dialkylimidazole and ionic liquid, which is applied in the preparation of carboxylate, carboxylate, sulfonate, etc., which can solve the problem of high specificity of solvent, reduction of production efficiency of ionic liquid, instability of methyl carbonate anion, etc. problems, to achieve the effect of improving purity, reducing the amount of reaction solvent used, and improving production efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] (1) In the autoclave, add N-ethylimidazole 41.05g (0.50mol), dimethyl carbonate 67.56g (0.75mol) and solvent ethylene glycol 86.89g (solvent addition amount is N-ethylimidazole and carbonic acid two 0.8 times the total mass of methyl ester), close the autoclave, replace with nitrogen for 6 min, then heat to 130 ° C, stir at 500 r / min, monitor the reaction progress by GC, after 32 h of reaction, no N-ethylimidazole can be detected, stop the reaction, and obtain 186.71 g of 46.5% pale yellow 1-ethyl-3-methylimidazole carbonate monomethyl carbonate solution in alcohol.
[0029] (2) 40.04g of 1-ethyl-3-methylimidazole monomethyl carbonate alcohol solution with a content of 46.5% and 15.31g of trifluoromethanesulfonic acid with a content of 98.0% were added to the reaction flask, and after stirring at 60°C for 4 hours, the solution The pH value remained unchanged, the reaction was stopped, the solvent ethylene glycol was evaporated under reduced pressure at 90 °C, and then m...
Embodiment 2
[0031] (1) 41.05g (0.50mol) of N-ethylimidazole, 58.55g (0.65mol) of dimethyl carbonate and 109.56g of solvent 1,4-butanediol (the amount of solvent added is N-ethyl) were added to the autoclave 1.1 times the total mass of imidazole and dimethyl carbonate), close the autoclave, replace with nitrogen for 6 min, then heat to 135 ° C, stir at 500 r / min, monitor the reaction progress by GC, after 30 h of reaction, no N-ethylimidazole can be detected, The reaction was stopped to obtain 195.66 g of a light yellow 1-ethyl-3-methylimidazole carbonate monomethyl carbonate alcoholic solution with a content of 43.71%.
[0032] (2) 42.60g of 1-ethyl-3-methylimidazole carbonate monomethyl carbonate alcohol solution with a content of 43.71% and 6.06g of acetic acid with a content of 99.0% were added to the reaction flask, and the pH value of the solution remained unchanged after stirring at 60°C for 6 hours. , stop the reaction, evaporate the solvent 1,4-butanediol under reduced pressure at...
Embodiment 3
[0034](1) N-butylimidazole 49.67g (0.4mol), dimethyl carbonate 46.84g (0.52mol) and solvent 3-methyl-1,3-butanediol 86.86g (solvent addition amount) were added to the autoclave is 0.9 times the total mass of N-butylimidazole and dimethyl carbonate), close the autoclave, replace with nitrogen for 6 minutes, then heat to 140 ° C, stir at 500 r / min, monitor the reaction progress by GC, after 48 hours of reaction, no N can be detected -Butylimidazole, stop the reaction, and obtain 171.23 1-butyl-3-methylimidazole monomethyl carbonate alcoholic solution with a content of 45.98%.
[0035] (2) 46.60g of 1-butyl-3-methylimidazole carbonate monomethyl carbonate alcohol solution with a content of 45.98% and 21.95g of tetrafluoroboric acid with a content of 40.0% were added to the reaction flask, and after stirring at 75°C for 6 hours, the pH of the solution The value remains unchanged, the reaction is stopped, the solvent 3-methyl-1,3-butanediol is evaporated under reduced pressure at 1...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com