Compound preparation prepared from clindamycin and cimetidine and preparation method
A technology of clindamycin and compound preparations, which is applied in the field of medicine, can solve problems such as adverse reactions and poor curative effect of patients, and achieve the effects of enhancing curative effect, improving body immunity, and taking conveniently
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] A compound formulation prepared by clindamycin and cimetidine, in the active ingredient of the compound preparation, the weight ratio of clindamycin and cimetidine is 0.075: 0.05, and the allowance is an excipient. The preparation method comprises the following steps:
[0022] (1) Weigh 75g of clindamycin API and 50g of cimetidine API, and spare;
[0023] (2) Take the appropriate amount of the required excipients suitable for the preparation of dry suspension and set aside;
[0024] (3) According to the preparation method of pharmacological dry suspension, the dry suspension is made, so that each generation contains clindamycin 150mg and cimetidine 100mg.
Embodiment 2
[0026] A compound formulation prepared by clindamycin and cimetidine, in which the weight ratio of clindamycin and cimetidine is 0.30: 0.20, and the residual amount is an excipient. The preparation method comprises the following steps:
[0027] (1) Weigh 30g of clindamycin API and 20g of cimetidine API, and set aside;
[0028] (2) Take the appropriate amount of the required excipients suitable for the preparation of the capsule and set aside;
[0029] (3) Make capsules according to the preparation method of pharmacy capsules, so that each capsule contains clindamycin 300mg and cimetidine 200mg.
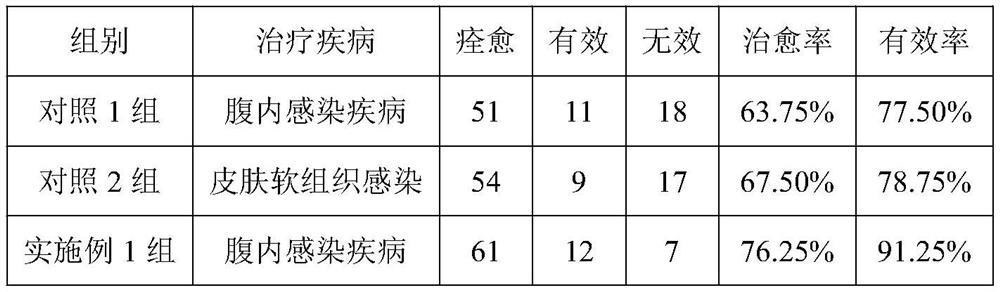
[0030] Efficacy test
[0031] 1. Select the drugs prepared by Examples 1 and 2 and conduct clinical trials.
[0032] According to the parallel, randomized, controlled experimental design scheme, a control group is strictly set up to observe the clinical efficacy of the drugs prepared in Examples 1 and 2.
[0033] 2. Pathological selection
[0034] Inclusion of cases: Adult patients diagnos...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com