Derivatization reagent, synthesis method thereof and method for in-situ analysis of monoamine neurotransmitters based on MALDI-MS (matrix-assisted laser desorption ionization-mass spectrometry)
A technology for derivatizing reagents and neurotransmitters, which is applied in the field of bioanalytical chemistry to achieve the effects of high efficiency, simple operation, and low preparation cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
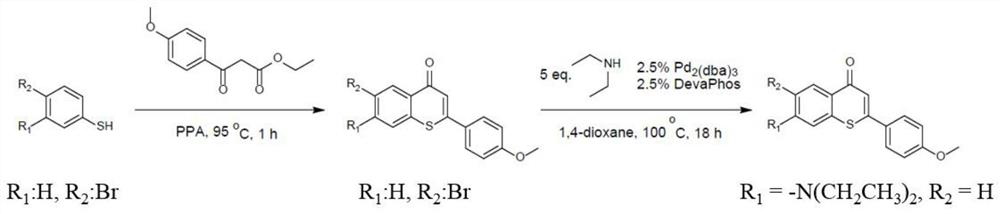
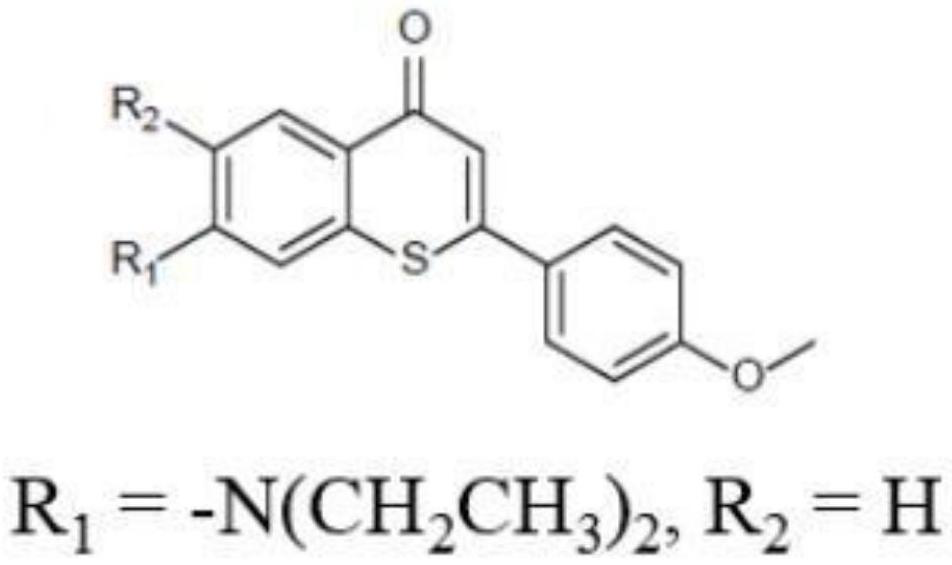
[0035] The synthetic route diagram of the derivatizing agent of the monoamine neurotransmitter compound of the present invention is as follows: figure 1 As shown, the specific synthesis method comprises the following steps:
[0036] Step 1) Polyphosphoric acid (PPA, 22 g), 3-bromothiophenol (2 g, 10.6 mmol) and ethyl 3-(4-methoxyphenyl)-3-oxopropionate were combined at 95°C (2.6 g, 11.66 mmol) of the mixture was stirred in a beaker for 2 h.
[0037] Step 2) After the mixture of step 1) was cooled to room temperature, ice water was added to quench the reaction.
[0038] Step 3) Extract three times with 100 mL of dichloromethane, and combine the extracts.
[0039] Step 4) use the organic extract in step 3) with Na 2 SO 4 It was dried, filtered and the crude product was further purified by flash column.
[0040]Step 5) The purified product (347 mg, 1 mmol) in step 4) was put into a test tube, and tridibenzylideneacetone dipalladium, (22.3 mg, 0.025 mmol), 2-dicyclohexylphosp...
Embodiment 2
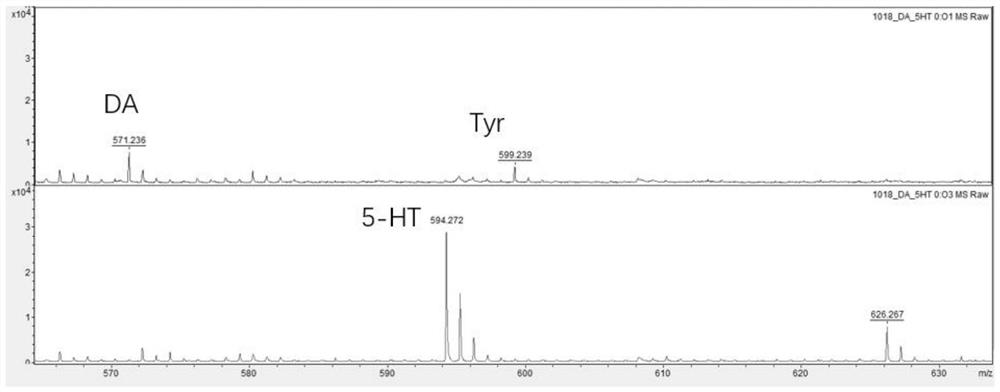
[0044] In this example, the brain tissue obtained from mice is used to detect amine neurotransmitters. The mass spectrometer is ultrafleXtreme and the MALDI-TOF / MS is from BRUKER. The concentration of the derivatization reagent prepared in Example 1 is 20mg / mL derivatization reagent solution, the detection includes the following steps:
[0045] Step 1. Add 300 mL of methanol to the mouse brain and homogenize it with a magnetic bead beater for 30 seconds.
[0046] Step 2. Centrifuge the homogenate at 10,000 rpm at 4° C. for 30 minutes, collect the supernatant and pass it through a 0.20 mm filter.
[0047] Step 3. Add derivatization reagent solution to the filtered supernatant, the reaction temperature is room temperature, and triethylamine is used to adjust the pH to 8-10, and then directly used for matrix-assisted laser desorption ionization time-of-flight (MALDI-TOF- MS) analysis.
[0048] Among them, the analysis conditions of matrix-assisted laser desorption ionization ti...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com