Imidazopyridine derivative and application thereof
A technology of imidazo and pyridine, which is applied in the field of medicine and can solve problems such as drug restrictions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
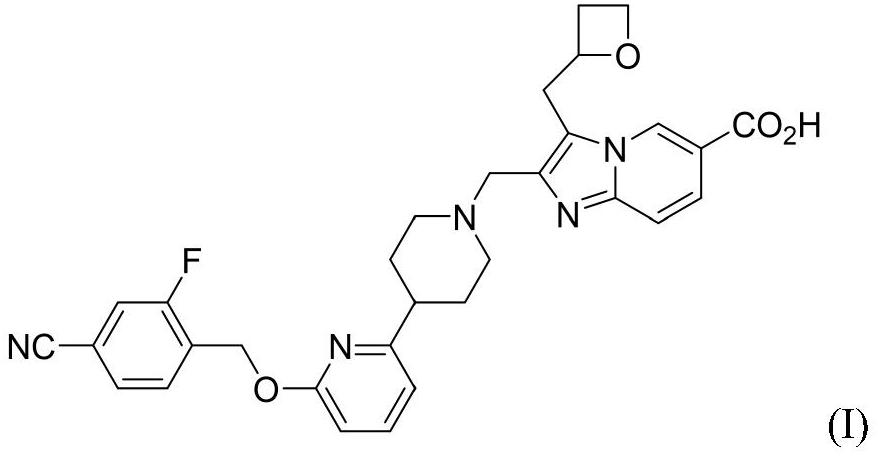
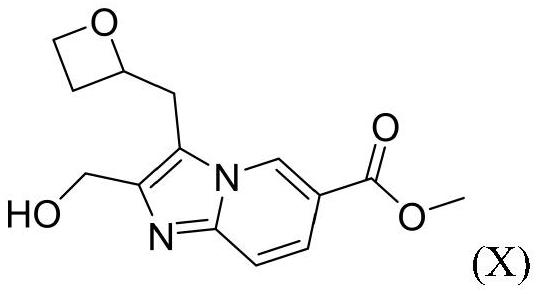
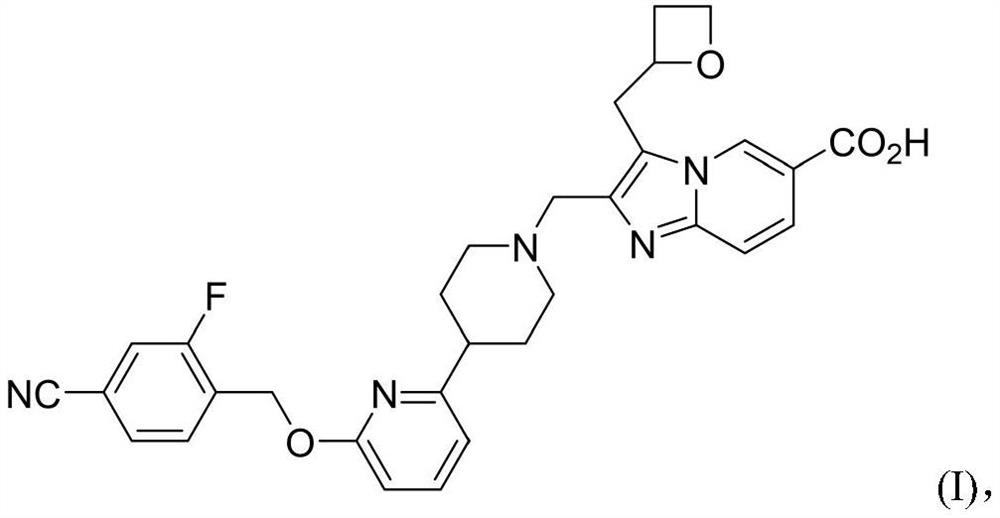
[0095] Compound of formula I 2-((4-(6-((4-cyano-2-fluorobenzyl)oxy)pyridin-2-yl)piperidin-1-yl)methyl)-3-(oxa Preparation method of cyclobutan-2-ylmethyl)imidazo[1,2-a]pyridine-6-carboxylic acid (hereinafter referred to as "compound 1")
[0096]
[0097] (1) Preparation of compound 1-2
[0098]
[0099] Compound 1-1 (20.0 g, 181.8 mmol) and TBSCl (27.4 g, 181.8 mmol) were added to DMF (300 mL) followed by imidazole (12.3 g, 181.8 mmol) and DMAP (5.5 g, 45.4 mmol), respectively. Under a nitrogen atmosphere, the reaction solution was reacted at room temperature for 16 hours. join H 2 O (500 mL), the reaction solution was extracted with EtOAc (3 x 500 mL). with H 2 The combined organic phases were washed with O (3 x 500 mL) and brine (400 mL), dried (Na 2 SO 4 ), filtered and concentrated. Flash chromatography (SiO 2 , 10% EtOAc-hexane) to give compound 1-2 (35.5 g, 86.7%), [ 1 H NMR(400MHz,DMSO-d6)δ5.13(d,J=5.1Hz,1H),3.68-3.45(m,5H),0.84-0.80(m,9H),0.02-0.03(m,6H)...
Embodiment 2
[0141] 2-((4-(6-((4-cyano-2-fluorobenzyl)oxy)pyridin-2-yl)piperidin-1-yl)methyl)-3-(morpholinomethyl) ) Preparation method of imidazo[1,2-a]pyridine-6-carboxylic acid (compound 2)
[0142]
[0143] (1) Preparation of compound 2-2
[0144]
[0145] The flask containing dry DMF (50 mL) was cooled to 0°C and phosphorous oxychloride (8.5 mL, 93 mmol) was added. The cooling bath was removed and the solution was stirred at room temperature for 1 hour. The red reaction mixture was treated with a solution of ethyl 6-bromoimidazo[1,2-a]pyridine-2-carboxylate (10 g, 37.2 mmol) in dry DMF (50 mL) and after 3 h the mixture was heated to 60 °C , the reaction mixture was poured into ice water (100 mL), and extracted with dichloromethane (3 x 80 mL) by adding 2N aqueous sodium hydroxide solution. The combined organic phases were dried over sodium sulfate and concentrated in vacuo to give compound 2-2 (5.6 g, 34%), [ 1 H NMR (400MHz, CDCl 3 )δ10.66(s,1H),9.83(dd,J=1.9,0.8Hz,1H),7.7...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com