Reverse transcriptase mutant capable of improving thermal stability and inhibitor tolerance and application of reverse transcriptase mutant
A technology of reverse transcriptase and thermal stability, applied in the biological field, can solve problems such as high price and achieve the effects of improving thermal stability, high thermal stability, and improving inhibitor tolerance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
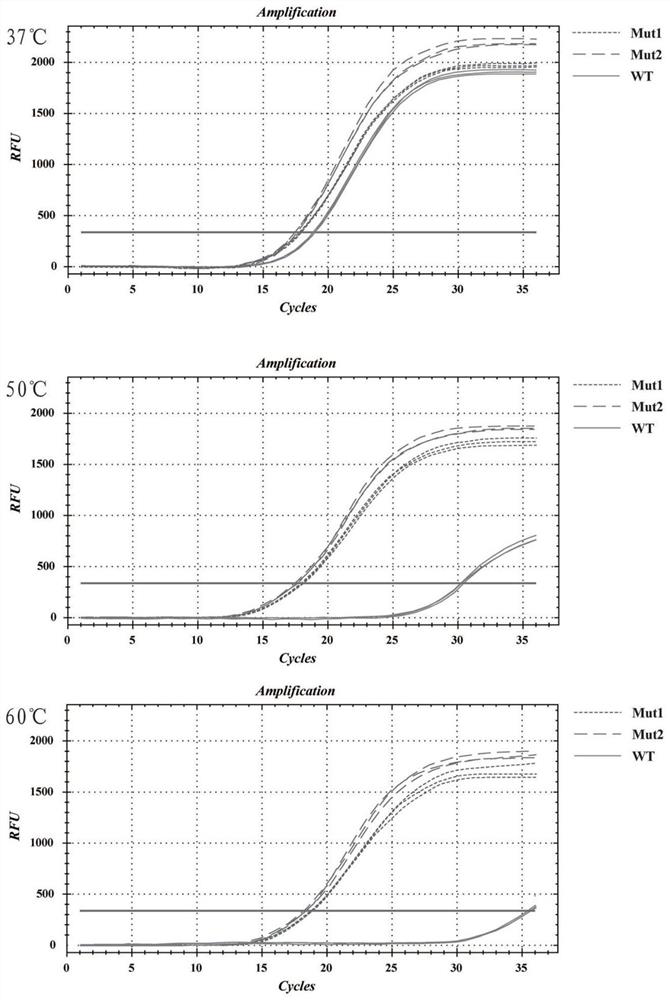
[0027] Mutant thermostability identification
[0028] Using SEQ ID NO. 1 as a template, using conventional site-directed mutagenesis or gene synthesis, and protein recombinant expression and purification methods, two M-MLV reverse transcriptase mutants containing different combinations of mutation sites were obtained respectively. One contains 5 mutation sites including L139P, T197A, G308C, D524G and L603W and is named Mut1; the other contains 6 mutation sites including L139P, T197A, D209A, G308C, D524G and L603W and is named Mut2.
[0029] The above mutants Mut1 and Mut2 were selected respectively. At the same time, wild-type M-MLV reverse transcriptase was used as the control, 1 μg of extracted human total RNA was used as the template, and Oligo(dT) was used as the template. 20 For the reverse transcription primers, the reverse transcription reaction system was prepared according to Table 1, and the reaction was carried out at 37°C, 50°C, and 60°C for 30 min respectively. T...
Embodiment 2
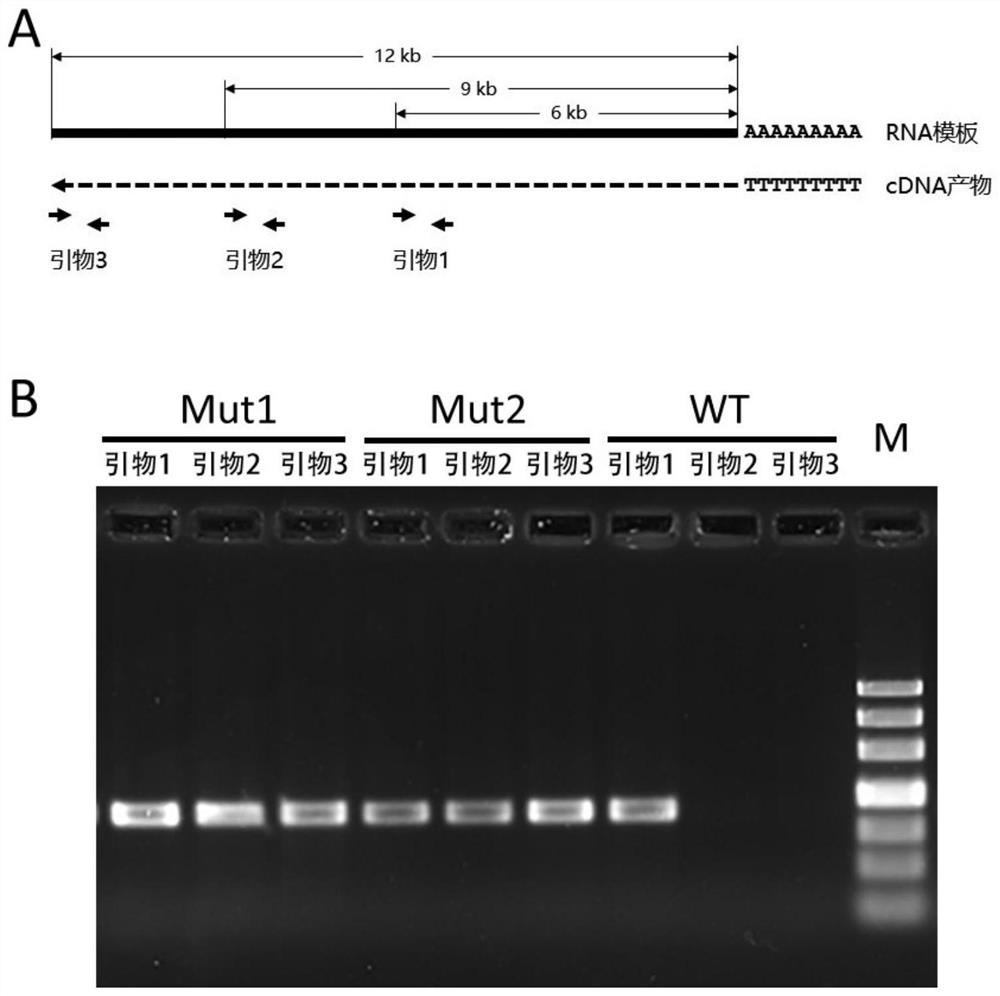
[0031] Identification of mutant processivity
[0032] Mutants Mut1 and Mut2 were taken respectively, 1 μg of human total RNA was used as the template, and Oligo(dT) was used as the template. 20 For the reverse transcription primers, the reverse transcription reaction system was prepared according to Table 1, and the reaction was carried out at 50 °C for 30 min; at the same time, the wild-type M-MLV reverse transcriptase was used as a control, and the reaction was carried out at 37 °C for 30 min. Then take about 100ng of cDNA products each, take the human HERC1 gene cDNA as a template, and use three pairs of primers (SEQ ID No. 4-9) with a distance of 6kb, 9kb and 12kb from the reverse transcription start site to carry out PCR amplification. The program is: pre-denaturation at 94°C for 3 min, followed by 35 cycles of 94°C for 30s, 60°C for 30s, and 72°C for 20s ( figure 2 A), use agarose gel electrophoresis to judge whether the length of the synthesized cDNA reaches 6kb, 9kb ...
Embodiment 3
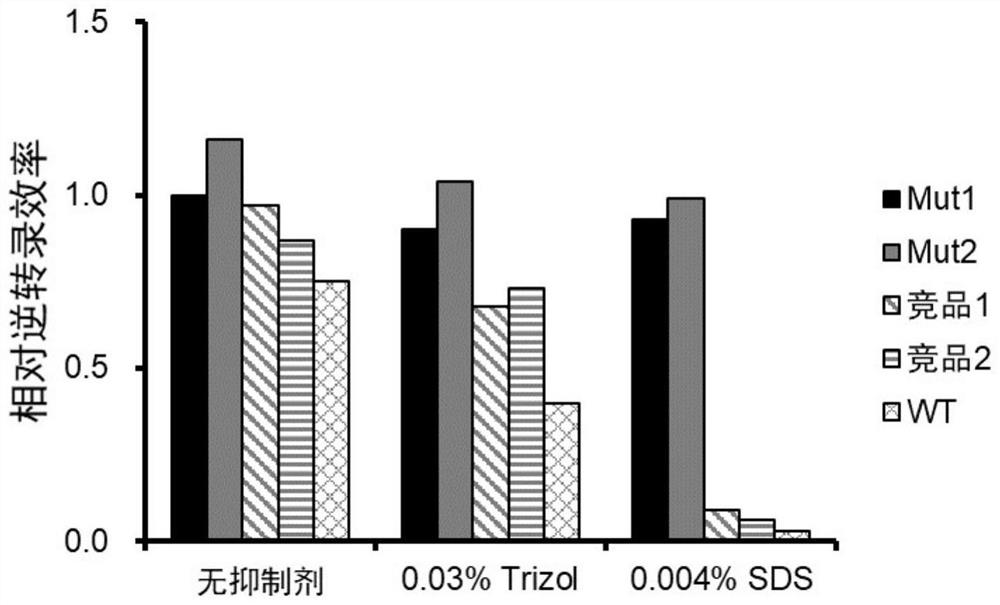
[0034] Identification of mutant inhibitor tolerance
[0035] Take mutant Mut1 and Mut2 respectively (optimum reaction temperature 50 ℃), at the same time wild-type M-MLV reverse transcriptase (optimum reaction temperature 37 ℃) and purchased commercial HiScript II Reverse Transcriptase (competing product 1, the most The optimum reaction temperature was 50°C) and TransScript Uni Reverse Transcriptase (competing product 2, the optimum reaction temperature was 50°C) as the control, 1 μg of human total RNA was used as the template, and Oligo(dT) 20For reverse transcription primers, prepare a reverse transcription reaction system according to Table 1, in which Trizol (Merck, Item No. T9424) was additionally added to the reaction system, with a final concentration of 0.03%; or SDS (Aladdin, Item No. S108349), with a final concentration of 0.004%, Then react at the respective optimum temperature for 30 min. cDNA products were detected by real-time quantitative PCR using GAPDH gene-s...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com