Synthesis method of peptide nucleic acid guanine monomer
A synthesis method and guanine technology are applied in the field of synthesis of peptide nucleic acid guanine monomers, which can solve the problems of long synthesis process route, difficult reaction yield, increase of by-products, etc., so as to reduce the types of reaction raw materials and simplify the synthesis process. Simple and convenient effects for routing and post-processing
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
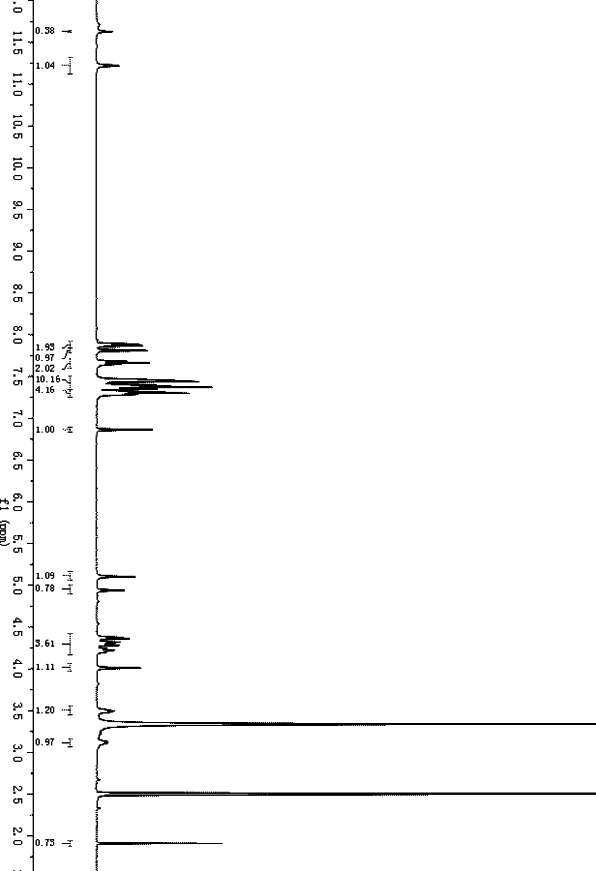
[0030] A synthetic method of peptide nucleic acid guanine monomer, comprising the steps:
[0031] 1) Dissolve 41.9 g of 2-N-(diphenylmethoxycarbonyl)guanine-9-acetic acid in 200 ml of N,N-dimethylformamide, add 11.5 g of N-hydroxysuccinimide with stirring , 57.6 grams of 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride was added at 0°C, and the reaction was stirred at room temperature. TLC showed that the reaction was completed, and the precipitated solid was filtered and washed with water once. to obtain 2-N-(diphenylmethoxycarbonyl)guanine-9-acetic acid NHS active ester, which is directly used in the next step without further purification, and the mother liquor is distilled under reduced pressure to recover the solvent;
[0032] 2) Dissolve 34 g of N-(2-Fmoc-aminoethyl)glycine in 200 ml of N,N-dimethylformamide, add the NHS active ester obtained in step 1), and dropwise add 12.9 g of N at 0°C , N-diisopropylethylamine, slowly rise to room temperature, react overn...
Embodiment 2
[0036] A synthetic method of peptide nucleic acid guanine monomer, comprising the steps:
[0037] 1) Dissolve 41.9 g of 2-N-(diphenylmethoxycarbonyl)guanine-9-acetic acid in 150 ml of N,N-dimethylformamide, add 12.5 g of N-hydroxysuccinimide with stirring , 57.6 grams of 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride was added at 0°C, and the reaction was stirred at room temperature. TLC showed that the reaction was completed, and the precipitated solid was filtered and washed with water once. to obtain 2-N-(diphenylmethoxycarbonyl)guanine-9-acetic acid NHS active ester, which is directly used in the next step without further purification, and the mother liquor is distilled under reduced pressure to recover the solvent;
[0038] 2) Dissolve 34 g of N-(2-Fmoc-aminoethyl)glycine in 150 ml of N,N-dimethylformamide, add the NHS active ester obtained in step 1), and dropwise add 12.9 g of N at 0°C , N-diisopropylethylamine, slowly rise to room temperature, react overn...
Embodiment 3
[0040] A synthetic method of peptide nucleic acid guanine monomer, comprising the steps:
[0041] 1) Dissolve 41.9 g of 2-N-(diphenylmethoxycarbonyl)guanine-9-acetic acid in 150 ml of N,N-dimethylformamide, add 11.5 g of N-hydroxysuccinimide with stirring , 60.6 grams of 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride was added at 0°C, and the reaction was stirred at room temperature. TLC showed that the reaction was completed. The precipitated solid was filtered and washed with water once. to obtain 2-N-(diphenylmethoxycarbonyl)guanine-9-acetic acid NHS active ester, which is directly used in the next step without further purification, and the mother liquor is distilled under reduced pressure to recover the solvent;
[0042] 2) Dissolve 34 g of N-(2-Fmoc-aminoethyl)glycine in 150 ml of N,N-dimethylformamide, add the NHS active ester obtained in step 1), and dropwise add 12.9 g of N at 0°C , N-diisopropylethylamine, slowly rise to room temperature, react overnight...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com