Method for accurately and quantitatively regulating and controlling exocytosis level of escherichia coli recombinant protein and application
A technology of Escherichia coli and recombinant Escherichia coli, which is applied in the field of genetic engineering and fermentation engineering, can solve problems such as toxicity, achieve the effects of improving permeability, optimizing application effect, and promoting extracellular secretion
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0067] Example 1 Knockout of tryptophan synthase gene cluster trpEDCBA in recombinant Escherichia coli
[0068] (1) Using plasmid pKD13 as a template, design primers and PCR amplify the homologous fragment containing the Kan resistance gene for replacing the trpEDCAB sequence, wherein the Kan resistance gene contains FRT sites on both sides to obtain the trpEDCBA sequence integration frame fragment.
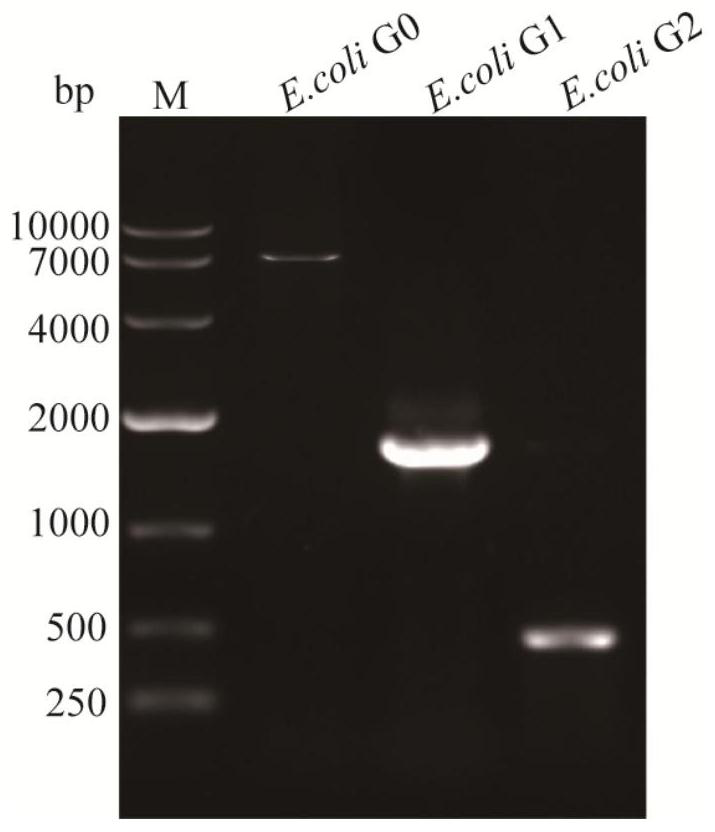
[0069](2) Transform the pKD46 plasmid into E.coli BL21(DE3) competent cells to obtain E.coli G0 strain, prepare electrotransformed competent cells, and electro-transform the integration frame fragment obtained in step (1) into E.coli In the G0 competent state, the transformation solution was post-cultured and then coated with LB solid medium containing kanamycin (Kan) and ampicillin to obtain transformant BL21::kan, using primers TrpA-pKD13-FW and TrpE- pKD13-RW was verified by colony PCR amplification to verify whether the trpEDCBA sequence was successfully edited, and the posit...
Embodiment 2
[0073] Example 2 Screening and characterization of stationary phase promoters
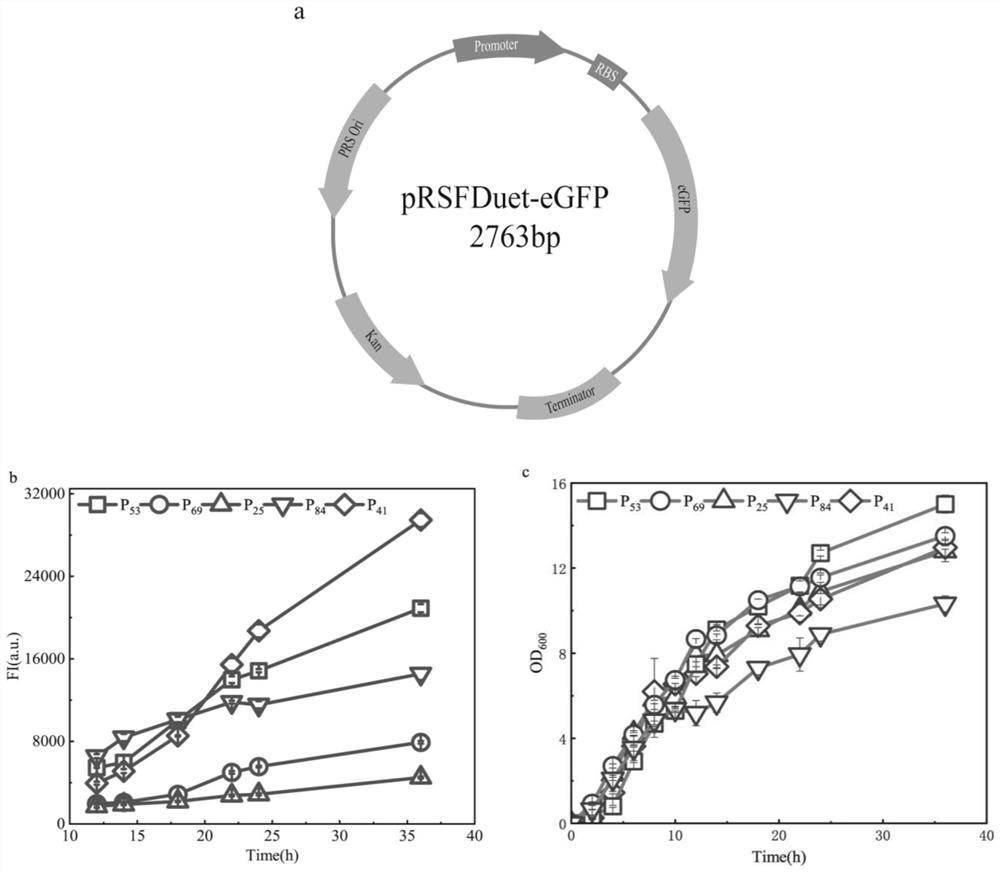
[0074] A library of 40 promoters was synthesized (a partial list of promoters is shown in 1) for screening different target promoters with gradient expression levels. Firstly, the lacI gene fragment corresponding to the lac operon on the plasmid pRSFDuet-1 was deleted to obtain the plasmid pRSFDuet-1-ΔlacI. The 40 promoters in the promoter library were used to replace the original T7 promoter in the plasmid pRSFDuet-1-ΔlacI respectively to construct a plasmid library containing 40 different promoters. Using green fluorescent protein (eGFP) as a model protein, different promoters with gradient expression levels were screened. Screening to obtain 5 different eGFP expression intensities (e.g. figure 2 ), and the protein expression level is concentrated in the promoter in the stationary phase (P 25 , P 41 , P 53 , P 69 , P 84 ) for the construction of novel regulatory systems in subsequent stud...
Embodiment 3
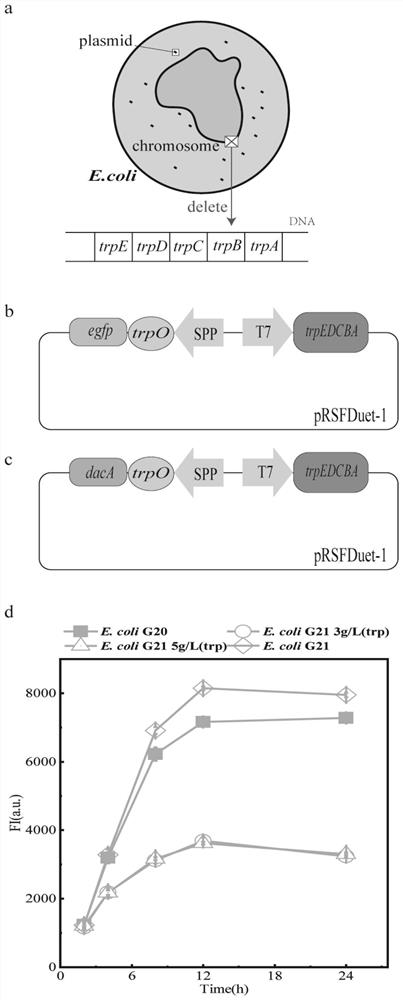
[0077] Example 3 Design and application of a new tryptophan operator system
[0078] Design of the new tryptophan operon system: The new tryptophan operon system consists of E. coli cells knocked out of the tryptophan synthase gene cluster trpEDCBA and a recombinant plasmid; the recombinant plasmid contains T7 promoter, SPP-type promoter, Tryptophan synthase gene cluster trpEDCBA, operator region trpO and D,D-carboxypeptidase gene dacA; the operator region trpO has a binding site that can bind to a covalent dimer formed by the repressor protein trpR and tryptophan The T7 promoter regulates the expression of trpEDCBA; the SPP regulates the gene expression of the operator region trpO; the downstream of the operator region trpO is connected to the dacA gene; the transcription directions of the T7 promoter and promoter SPP are opposite; the The nucleotide sequence of the T7 promoter is shown in SEQ ID No. 6; the nucleotide sequence of the SPP type promoter is shown in SEQ ID No. 7...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com