Escherichia coli recombinant strain as well as preparation method and application thereof
A technology of recombinant strains and Escherichia coli, which is applied in the field of biomedicine, can solve the problems of ompF protein excretion outside the cell, ompC potential function or absence, micF regulatory function loss, etc., to achieve the effect of increasing expression and secretion efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0056] Example 1 Construction of Escherichia coli Recombinant Strain
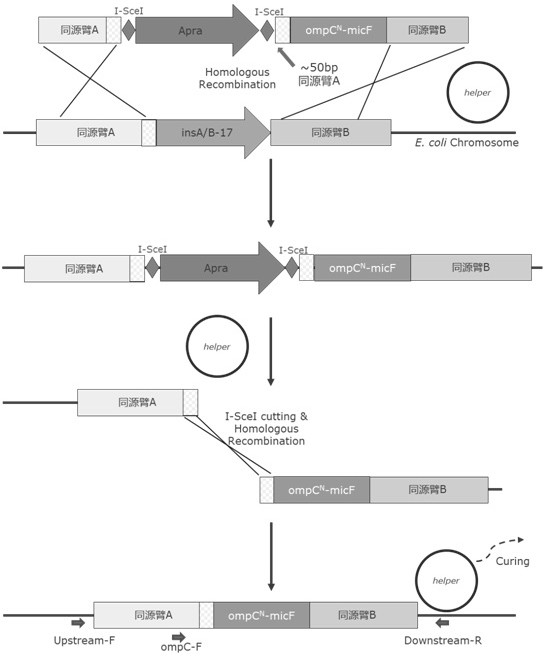
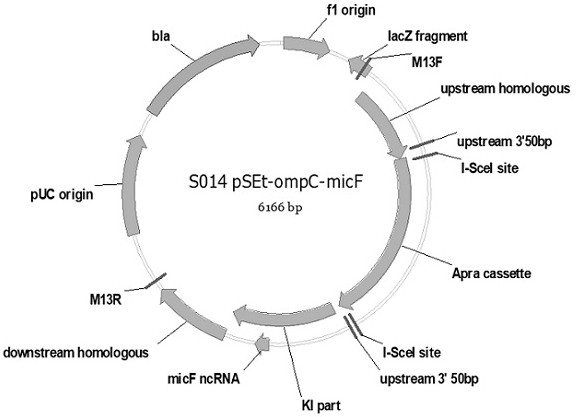
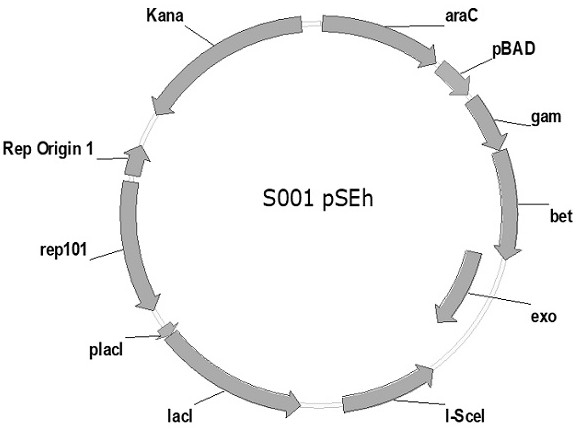
[0057] In this study, through the sequence alignment of strain K and strain B, it was found that insA / B-17 was inserted into the ompC-micF site in strain B, resulting in the deletion of 56 amino acid coding sequences from the N-terminal of ompC gene. The promoter and downstream rcsD and rcsB genes are deleted, and the 56 amino acid sequence deleted from the N-terminus of the ompC protein contains a signal peptide (signal peptide) and a transmembrane βstrand that is crucial for anchoring ompC to the outer membrane, K strain ompC The protein expression sequence is shown in SEQ ID NO: 16, and the B strain ompC protein expression sequence is shown in SEQ ID NO: 17. Based on the above findings, the present invention transforms Escherichia coli B strain by scarless homologous recombination of I-sceI DNA endonuclease to obtain a new strain with ompC-micF gene locus repaired, and the method includes: 1) Knock out ...
Embodiment 2
[0092] Example 2 Application of the modified Escherichia coli B strain in the expression of foreign proteins
[0093] Recombinant protein S (GLP1(7-37) linked to an expressive fusion tag), recombinant protein P (PTH (teriparatide) linked to an expressive fusion tag) and recombinant protein F (Fc fragment of immunoglobulin IgG) will be expressed ) expression plasmids were transferred into SQLa (control group) and SQLb (recombinant strain) competent cells by heat shock method, and cultured in TB medium at 37°C to OD 600 was about 2.0, and IPTG (final concentration of 1 mM) was added to induce recombinant protein expression. After induction at 37°C overnight, the cells were collected by centrifugation, and the walls were broken by ultrasonic waves. SDS-PAGE electrophoresis analysis of whole protein, soluble protein and insoluble protein, the results are as follows Figure 6A As shown, the molecular weight of Protein S is 5154 Da, the molecular weight of Protein P is 5888 Da, an...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com